Abstract
Background
Antimicrobial resistance (AMR) is a global health threat affecting treatment outcome in animals and humans. A pre-requisite for development of AMR reduction strategies is knowledge of antimicrobial use patterns, and how these affect resistance development. The aim of this study was to determine antimicrobial usage (AMU) and whether such usage was associated with AMR in Salmonella from poultry farms in Northwest Nigeria.
Results
Fifteen (37%) of antimicrobial products observed contained compounds that are of highest priority and critically important for human medicine. Broilers chicken consumed higher (28 ± 14 mg/kg active ingredients) amounts of antimicrobials compared to layers (13 ± 8 mg/kg) per week (p = 0.0009). Surprisingly, chickens raised under backyard system consumed higher amounts of antimicrobials (34 ± 7 mg/kg) than poultry in other systems (p = 0.02). High levels of resistance to tetracycline (58%), sulphonamides (65%), ciprofloxacin (46%) and gentamicin (42%) correlated with high farm level usage of these antimicrobials, and there was a strong correlation (r = 0.9) between farm usage and resistance of isolates to the same antimicrobials (p = 0.03).
Conclusion
High AMU, including use of highest priority critically important antimicrobials was observed at poultry farms in Northwest Nigeria. AMU correlated with high levels of resistance. Communication of prudent use of antimicrobials to farmers and regulation to obtain reduction in AMU should be a priority.
Background
In 2015, the World Health Organization (WHO) launched its Global Action Plan on antimicrobial resistance (AMR) with one of its key objectives being the development and enhancement of monitoring systems for antimicrobial usage (AMU) worldwide [1]. The World Organization for Animal Health (OIE) also recognises that antimicrobial resistance is a global public and animal health concern that is influenced by the use of antimicrobial agents in both humans and animals, and it is suggested that OIE member-countries adopt international standards on the prudent use of antimicrobial agents and monitor antimicrobial usage in food producing animals [2]. However, measuring AMU in animal production in low- and middle-income countries (LMICs) is challenging due to the large numbers of small-scale farming units, access to antimicrobials sold “over the counter,” and generally poorly enforced regulatory frameworks [3].
Antimicrobials are used in livestock production mainly to prevent and control diseases, but also as growth promoters and to protect animal welfare [4, 5]. Because of global increase in livestock production, AMU in animal production has been predicted to increase by 11.5% from 2017 to 2030 [6] mostly driven by increased animal protein consumption in LMICs [3].
AMU on farms selects for AMR bacteria and genetic determinants of resistance may spread to humans either through direct contact to livestock, consumption of contaminated foods or indirectly through environmental pathways, leading to reduced efficacy of treatment in both animals and humans [7, 8]. Since the initial observation on the potential link between veterinary use of antimicrobials (AMs) and AMR in bacteria [9], the link has been confirmed in several studies describing the association between AMU and AMR, using combined AMU/AMR surveillance data [10,11,12].
As part of the recommendation by OIE, studies targeted to evaluate antimicrobial usage in food animals should include information about the usage pattern by species of animal and antimicrobials used in farms for a specific period of time [2]. In Nigeria and neighbouring Cameroun, high usage of antimicrobials has been reported in poultry farms by qualitative assessments using structured questionnaires [13, 14], but these data were not evaluated and correlated to AMR levels, and the validity of the AMU data was not confirmed by real observations on farms. The aim of the present study was to investigate antimicrobial usage in poultry farms in Nigeria, and to determine how such usage correlated with data on AMR in Salmonella spp. isolated from the same farms.
Results
Types of antimicrobials used at farms
A total of 41 antimicrobial-containing products were observed on the farms. Of these, 36 products contained antimicrobial active ingredients (AAIs) only, while five also contained other substances such as vitamins. Twenty products contained two or more antimicrobials, 39 products were intended for oral use, and 34 products were in powdered form (Table 1).
Twenty antimicrobial types were declared in the 41 antimicrobial preparations (Additional file 1). The most commonly-used antimicrobials were: doxycycline, oxytetracyclines, colistin and erythromycin which were used on 28 (68%), 22 (54%), 22 (54%) and 21 (51%) of farms, respectively.
The aminoglycosides neomycin, gentamicin and streptomycin were commonly used at 13 (32%), 15 (37%) and 15 (37%) of farms respectively. There was considerably lower usage of penicillin (2%) and furazolidone (2%) than other drugs (Table 2). Thirty-two (78.1%) of the products contained AAIs that are classified as critically important for human medicine i.e. erythromycin, colistin, quinolones, aminoglycosides, oxazolidone and penicillin. Among these, 15 (37%) products contained AAIs that are categorised as “Highest priority critically important” namely colistin and ciprofloxacin as listed by WHO [15] (Table 3).
Quantification of antimicrobial usage
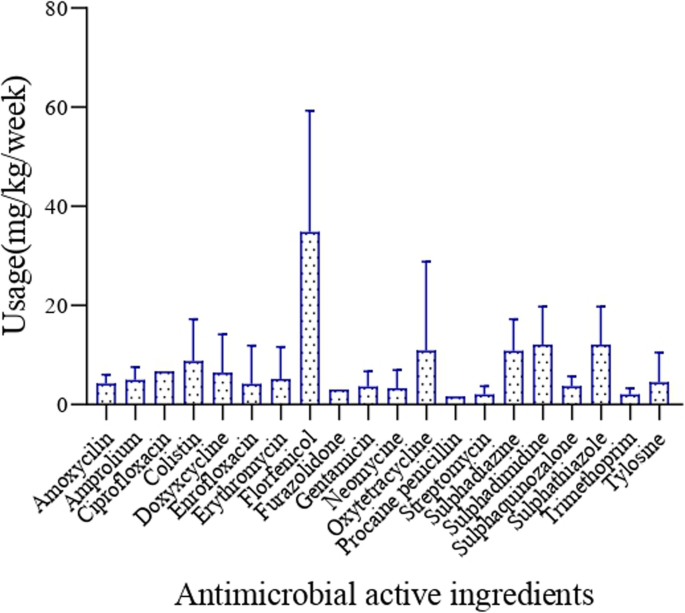
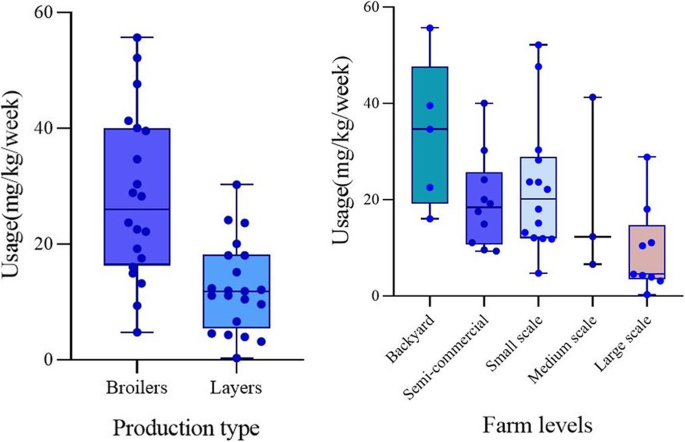
Florfenicol had the highest average usage per chicken per week (15 mg), followed by sulphadimidine, sulphathiazole (5 mg each), oxytetracycline (5 mg), colistin (4 mg) and ciprofloxacin (3 mg), while tylosine, (2 mg), gentamicin (2 mg), neomycin (2 mg), trimethoprim (1 mg), and streptomycin (1 mg) were the ones used the less (Fig. 1). The estimated weight of broilers after the growth period of 6 weeks was 2.5 kg, while that of layers after 24 weeks was 1.8 kg. Based on this, broiler chickens consumed higher amounts of antimicrobial active ingredients (28 ± 14 mg/kg per week) than layers (13 ± 8 mg/kg per week) (p = 0.0009). Backyard chicken was found to consume more antimicrobials (34 ± 7 mg/kg per week) than chicken raised in other production systems (p = 0.02) (Fig. 2).
Correlation between AMU and AMR
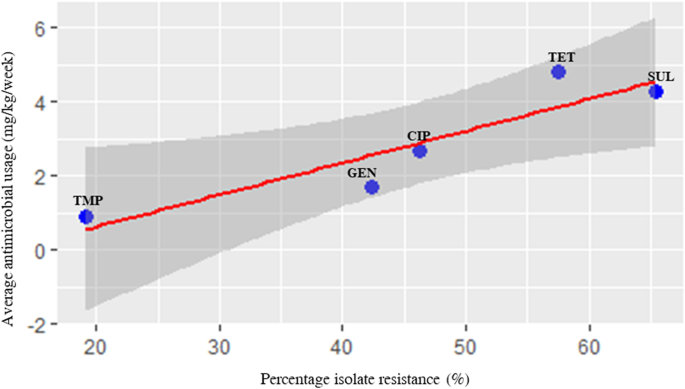
Antimicrobial resistance data for Salmonella isolated from the 41 farms were obtained from a previous study published by the same authors as this study [16]. Details of these isolates are shown in Additional file 2. There was a maximum of 30 and 120 days between Salmonella isolation and recordings of AMU use for broiler and layer farms, respectively. Five antimicrobial agents that were highly used in the farms and were part of the panel of antimicrobials used for susceptibility testing, were used to evaluate correlation between farm AMU and phenotypic resistance as well as correlation between AMU and prevalence of antimicrobial resistance genes (ARGs) conferring resistance to these antimicrobials . The results showed that high level usage of tetracycline and sulphonamides correlated with high level of isolates showing resistance to these antibiotics. Likewise, moderate usage of gentamicin and ciprofloxacin correlated with moderate percentage of Salmonella isolates being resistant to the same antimicrobials (r = 0.9, [CI = 0.2–0.99], p value = 0.03), and low farm usage of trimethoprim correlated with a low number of isolates being resistant to this drug (Table 4). Similarly, there was strong correlation between usage and percentage of isolates that had resistant genes conferring resistance to the antimicrobials (r = 0.9, [CI = − 0.004-0.99] p = 0.05). However, this was not statistically significant. Scatter plot analysis of the resistance and usage variables also showed uphill trends, indicating a positive strong relationship between these two variables (r = 0.9, p = 0.03) (Fig. 3).
Scatter plot analysis indicating positive correlation between percentage of isolate resistance and average usage of active antimicrobial ingredients of selected antimicrobials. Each dot represents a specific antimicrobial. The blue colour represents the 95% confidence interval. CIP, ciprofloxacin; GEN, gentamicin; SUL, sulphonamides; TET, tetracycline; and TMP, trimethoprim
Farms were categorized into high users and low users of antimicrobials (see Materials and Methods section), and data was used as inputs in modelling risk factors for presence of AMR in Salmonella at the farm. From the univariate analysis, magnitude of AMU and farm category was observed to have statistical significance on the resistance level of Salmonella isolates, while production type (broilers vs. layers) showed no significance, and this factor was therefore excluded in the logistic regression analysis. This observation made sense, since the interpretation was that high use of antimicrobials had the same effect in the two types of productions. In the second step, logistic regression analysis was used to evaluate if the magnitude of AMU could predict isolate resistance at farm level. From the logit equation, the results indicated that no AMU was associated with little resistance with a log odds of resistance equal − 0.4. There was a significant difference in the log odds of AMR by 2.8 between farms with low and high AMU. With the exception of large-scale farms, the log odds of AMR increased with an increase in flock size, however, the difference was not found to be statistically significant (Table 5).
Discussion
This study assessed type of antimicrobials used in poultry farms in Northwest Nigeria, quantified antimicrobial usage (AMU) in all categories of commercial poultry farms, and furthermore analysed for associations between AMU and levels of resistance (AMR) in Salmonella isolated in those farms.
Oral treatment was the most frequently used route of antimicrobial administration in poultry. About 95.1% of the products were oral formulations, and in 98.1% of the farms visited, the preferred method for administration was mass medication via drinking water. This finding is in agreement with a previous study that reported 80% of drugs were administered in drinking water in poultry raised in the Abia State state of Nigeria [17]. Drug administration via feed was not used likely because it is difficult for farmers to add and mix antimicrobials into feed, but also because sick chickens will continue to drink, but have a lower feed intake [14]. Oral medication via water is the most preferred route of drug administration of poultry, purposely because whole flocks can be treated over long periods. Consequently, large number of chickens, and their proximal environment, will experience long-term exposure to many different classes of antimicrobials exerting a high pressure for selection of bacterial resistance. This is likely to lead to increased levels of resistance compared to treatment of smaller groups, however, this need to be further investigated. Surprisingly, single animal or small group treatment was reported to have no significant influence on antimicrobial resistance development in nursery pigs [18], probably because treated and untreated pigs mingle, and a similar situation may exist in poultry farm. A further problem linked to mass medication via water is the likelihood of inadvertent under dosing due to reduced bioavailability, e.g. by inhomogeneous mixtures, chemical degradation of the drug, adverse interactions with feed components, or other drugs, and reduced feed or water intake by the diseased animals [5]. Individual therapy with injectable antimicrobials was only reported in 4.9% of farms and was mostly administered by large-scale farms that adhered to a principle of treatment of sick chickens in an isolation pen.
Florfenicol, oxytetracycline, sulphonamides ciprofloxacin and gentamicin were the most frequently used antimicrobials. This finding corroborates recent findings from a qualitative survey conducted by Al-Mustapha et al. [19] that observed gentamicin, sulfonamide and quinolone-based antimicrobials as the most frequently administered antimicrobials in poultry in Kwara State, Nigeria. Another study reported that clotrimoxazole, neomycin, oxytetracycline and chloramphenicol were the most frequently used antimicrobials in Ile-Ife State, Nigeria [20]. With the exception of florfenicol, all the antimicrobials used in the sampled poultry farms belong to the same classes of antimicrobials used in human medicine. It is particularly worrisome that many products contained AAIs that are categorised as “Highest priority critically important” for human health as listed by WHO, including colistin, quinolones, and macrolides. The common use of quinolones and colistin in the study area is of particular concern as these antimicrobials constituted third line of drugs for treatment in human medicine [3]. A similar study conducted by Joosten et al. [21] reported that aminopenicillins, fluoroquinolones and tetracycline were the most frequently used antimicrobials in broilers in nine European countries and only 3 to 26% of drugs were of the “highest priority” group of drugs. In our study, ciprofloxacin and enrofloxacin (quinolones) were mostly found alone as suspension of active ingredients, while colistin and erythromycin were typically found in cocktails with other antimicrobials like trimethoprim, doxycycline, amoxicillin, sulphonamides, and oxytetracycline. Over 60% of the products contained more than one antimicrobial agent. This is similar to a previous report which found that about 60% of antimicrobial products used in small scale chicken farms in Mekong Delta of Vietnam contained multiple antimicrobial active ingredients [3]. The clinical considerations for multiple antimicrobial therapy is to cover the spectrum of potential pathogens during poly-microbial infections or in acute infections for which the responsible micro-organism or resistance profile of the pathogen is unknown [22]. Using different classes of antimicrobial drugs in combination might result in synergistic antimicrobial interactions that enhance the inhibitory effect [22]. Nonetheless, the general benefits of combination therapy compared with single or sequential administration of antimicrobials for treating bacterial infections have been difficult to conclusively demonstrate [23], and recent studies have demonstrated that combination therapy resulted in higher frequency of multi drug resistance in Pseudomonas aeruginosa than single drug treatment [24]. It was also observed that 5% of the farms used furazolidone even though this drug has been banned for use in Nigeria since 2017 [25]. The continued use of furazolidone illustrated the lack of compliance of poultry farmers to this regulation. A similar study reported 5% usage of banned nitrofurantoin in commercial chicken farm in Yaounde, Cameroun [14].
This study highlighted a high level of antimicrobial usage (mg/kg) per week across all the categories of farms that raised broilers and layers in the study area. As far as we were able to determine, commercial poultry feed producers do not incorporate antimicrobials in feed in Nigeria, and thus the study probably provides an overview of the total AMU in the sampled poultry farms. From the result, one can infer that farmers in the study area administer 421.5 mg (28.1 mg/kg) per chicken of antimicrobial agents to broiler chicken for a 6 weeks production period. This is even higher than the 158.2 mg per chicken of antimicrobial agents used to produce one broiler in Mekong Delta of Vietnam [26]. The high usage observed in this study could be linked to real or perceived higher prevalence of disease, the lack of government restriction and control on antimicrobial usage and inappropriate adherence to dosing intervals [27]. Qualitative studies conducted elsewhere in Nigeria and in Uganda, using questionnaire surveys, have reported a high usage of antimicrobials in poultry farms [28, 29]. A recent report used a combination of antimicrobial sales data, food animal census and countries meat consumption to project global antimicrobial use in food animals for 2017 and 2030 [6]. This study further estimated a projected increase of 11.5% from 93,309 t of active antimicrobial ingredients in 2017 to 104,079 t by 2030. In the mentioned report African countries used lower quantities of antimicrobials in 2017 compared to other continents, but the continent is expected to experience the highest increase in antimicrobial consumption (37%) by 2030. This increase is likely to be higher in Nigeria due to its increase in population [30], underlining the need to get AMU under better control in the country.
Farms that raised broilers used higher amount of antimicrobials compared to layer farms. The high usage in broilers may be attributed to the common practice of administering antimicrobials and vitamins at the beginning of production cycle. Additionally, broiler farmers have been known to use antimicrobials for growth promotion and feed efficiency [31]. Also an earlier study conducted by Filippitzi et al. [32] showed that AMU expressed as doses per unit of animal-time was highest in broiler production followed by pig and dairy. Similarly, studies conducted using various metrics to quantify antimicrobial usage in Mekong Delta in Vietnam reported high usage of antimicrobial in broiler chickens [3, 26], though this was not compared with layer birds. In some developed countries, broiler production is performed with low use of antimicrobials [33,34,35,36]. Production in such countries could form models for how to reduce AMU and subsequently AMR in LMIC, focusing on prevention of disease through biosecurity management and use of vaccines, rather than use of antimicrobials.
This study observed decreasing usage of antimicrobials as the farm sizes increased. Backyard poultry production systems with flock size less than 200, that constitute the majority of poultry industry in Nigeria [37], administered higher amounts of antimicrobials compered to larger farms. This outcome was surprising and could be due to the fact that most backyard farms do not have consulting veterinarians, lack of technical ability to administer antimicrobials correctly, lower loss tolerance capacity or a higher perception of risk of disease by household farm owners [26]. The situation analysis of AMR in Nigeria that preceded the country’s national action plan on antimicrobial resistance showed systematic misuse and over use of antibiotics in livestock production system putting local, national and global communities at risk [38].
The Salmonella isolates included in this study were tested for susceptibility to 11 antimicrobials [16]. We found a strong positive correlation between antimicrobial farm usages with percentage of antimicrobial resistance of the Salmonella isolates. Prediction of genes that conferred resistance to these antibiotics also correlated with AMU, although not at a significant level. This underscores the importance of reducing AMU as part of plans to reduce AMR, even though it remains to be demonstrated that this will lead to significant reduction of AMR for all drug types. A previous report demonstrated that the use of antimicrobial in livestock drives the evolution, prevalence, and dissemination of antimicrobial resistance in bacteria isolated from such food-producing animals [39]. Meta-analyses have found that the seven European countries with the highest use antimicrobials also had the highest levels of resistance [12]. In Japan, a significant correlation was reported between antimicrobial resistance in E. coli and AMU in cattle, pigs, and broiler and layer chickens [10]. In contrast, some studies do not find any relationship between AMU and AMR in livestock farms [40]. The direct relationship and significance observed between farm AMU and resistance should be interpreted with care, when extrapolating statistical significance for biological significance. However, the relationship observed provides an insight that development and spread of AMR due to imprudent usage of antimicrobial in poultry farms can occur.
Different antimicrobials do not have the same bio-availability, and they are not of equal importance with regards to weight and therapeutic activity due to difference in their potency. In the current study, we have assumed that drug presence in the target site, irrespective of the potency, influence development of resistance relatively to the weight of the chicken. Since we compare between high and low AMU at farms with aggregated resistance estimates across different antimicrobials, this may cause some inaccuracy. However, we believe the data clearly supports that magnitude of antimicrobial usage is associated with the likelihood of resistance development in gut-intestinal bacteria in a poultry farm, especially those that raised less than 200 chickens. In these farms, more antimicrobial types and higher usage in the magnitude of antimicrobials was observed compared to larger farms. Administration of cocktails of antimicrobials could subject pathogens to increased selection pressure and more likelihood of developing resistance.
Conclusion
A wide range of antimicrobial products containing cocktails of antimicrobial active ingredients were used in commercial poultry farms in Nigeria. Relatively higher usage of antimicrobial agents per chicken per unit time was observed in broilers farms compared to chickens in layer farm, and resistance in Salmonella isolates from poultry farms was associated with the magnitude of antimicrobial use in farms.
Methods
Study area
This study was conducted in Northwest Nigeria. The area was selected because of its large geographical size, high human population density, and large poultry production as previously described [41].
Study design
Forty-one farms were selected using a multistage technique [42] to include five categories of poultry farms (backyard, semi-commercial, small-scale, medium-scale and large-scale farms) to obtain data on AMU (Additional file 3). Typical commercial poultry farms in Nigeria are categorized into five groups based on the number of chicken raised, level of biosecurity and production output: Backyard farms (less than 200 birds), semi-commercial farms (200–999 birds), small-scale farms (1000–4999 birds), and medium-scale farms (5000–9999 birds) to large-scale farms (more than 10,000 birds). The backyard farms represent the majority of the farms sampled in this study as they constituted a large proportion of the poultry production in Nigeria [37]. An active longitudinal study design was used for 1.5 to 6 months for broilers and layers, respectively. Each selected poultry farm was paid three visits (2 and 8 weeks interval for broiler and layer farms, respectively) to obtain data about AMU and prevalence of Salmonella infection and degree of environmental contamination at the farm. In the first visit, written consent to participate was obtained from farmers, and they were informed on their liberty to withdraw from the study at any time. In addition, farmers were trained on how to document used antimicrobial products by putting all used sachets and other types of packet material in a plastic container and by recording the information on an AMU data sheet. For medium and large-scale farms, the AMU data sheets (Additional file 4) were further given to the consulting veterinarian to record information about product used. In the second visit, we evaluated the AMU data collected at the farm by ensuring that farmers adhered to the training provided on how to collect and register their AMU data. During the last visit, AMU data were collected and recorded for analysis. Information about antimicrobial products and their formulations including the commercial name, route of administration, antimicrobial composition and number of containers used was obtained. The Anatomical Therapeutic Chemical classification system for veterinary medicinal products (ATCvet) was used for antimicrobial drug identification [43].
Estimation of antimicrobial usage
The formula adapted by Carrique-Mas et al. [26] with little modification was used to estimate usage in mg/kg per week (Uwc milligrams). This was obtained by dividing the multiple of the number of used antimicrobial products (Np) and amount of each active antimicrobial ingredient contained in the products (Ur milligrams) by the multiple of the length of reporting period for that farm (t weeks) and number of chickens present in the farm (Nc chickens) at the initial visit date divided by the weight (W) kg of the chicken at the end of the study period. The weight of broilers after the growth period of 6 weeks was estimated to be 2.5 kg, while that of layers after 24 weeks was 1.8 kg.
Salmonella status of farm and antimicrobial resistance profiles of isolates
In a cross sectional survey, carried out in parallel to the current study, pooled samples of shoe socks of fresh faecal droppings and dust samples were collected from the same 41 commercial poultry farms yielding a total of 82 samples collected to determine the prevalence and antimicrobial resistance profile of strains [41] and the reader is referred to that study for a detailed description of methods.
Data management and statistical analysis
Data from farm AMU were entered into Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA) spreadsheet for descriptive statistical analysis and then exported to IBM SPSS statistic 26.0 for inferential analysis. One-way analysis of variance and student unpaired t-test was used to test for significance between AMU and categorical variables (location, type of bird and farm category). Data was exported into statistical software R for correlation and modelling using relevant installed packages (cor.test and lm function) [44]. Pearson moment correlation was used to check for correlation between usage and resistance in the isolated Salmonella. AMU at the farm was categorized into low and high usage based on a previous estimate of 26.4 mg usage per chicken per week (10.6 mg/kg of broilers) [25]. AMU less than 10.6 mg/kg was termed low, while usage above this cut-off value was considered as high. To investigate if usage could predict resistance, a two-step statistical approach was employed. In the first step, univariate analysis was used to check for association between resistance and variables (magnitude of usage, production type (broiler or layer) and farm category). In the second step, statistical significant predictors were selected for logistic regression. Logistic regression was used to evaluate if the total antimicrobial active ingredients (magnitude of usage) consumed per week at farm level could predict resistance found in Salmonella to antimicrobials used on the farm. This was done by fitting the relationship into the equation.
Where, y (outcome) is the AMR status of farms (resistance vs non-resistance), x is the predictor where x1 is the AMU status of a farm (high or low, based on the cut off value described above) and x2 is the the different farm type based on flock size) and a is the intercept and b is the slope.
Ggplot2 was used to create scatter plots. Values of p less than 0.05 were considered statistically significant.
Availability of data and materials
All data produced as part of the current study is shown in the main text and the supplementary files.
Abbreviations
- AAI:
- Antimicrobial active ingredients
- AMU:
- Antimicrobial usage
- AMR:
- Antimicrobial resistance
- ARGs:
- Antibiotic resistance genes
- LMIC:
- Low middle-income countries
- MDR:
- Multidrug resistance
- NAFDAC:
- National Agency for Food Drugs Administration and Control
- NCDC:
- Nigerian Centre for Disease Control and Prevention
- OIE:
- World Organization for Animal Health
- WHO:
- World Health Organization
References
- 1.
Anon. WHO Global Action Plan on Antimicrobial Resistance. 2015. Available online at: http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/Eng_OIE_List_antimicrobials_May2015.pdf.
- 2.
OIE. OIE standards, guidelines and resolution on antimicrobial resistance and the use of antimicrobial agents: International Office of Epizootics; 2015.
- 3.
Van Cuong N, Phu DH, Van NTB, Dinh Truong B, Kiet BT, Hien BV, et al. High-resolution monitoring of antimicrobial consumption in Vietnamese small-scale chicken farms highlights discrepancies between study metrics. Front Vet Sci. 2019;6:1–13. https://doi.org/10.3389/fvets.2019.00174.
- 4.
Page SW, Gautier P. Use of antimicrobial agents in livestock. OIE Rev Sci Tech. 2012;31:145.
- 5.
Ungemach FR, Muller-Bahrdt D, Abraham G. Guidelines for prudent use of antimicrobials and their implications on antibiotic usage in veterinary medicine. Int J Med Microbiol. 2006;296:33–8. https://doi.org/10.1016/j.ijmm.2006.01.059.
- 6.
Tiseo K, Huber L, Gilbert M, Robinson TP, Van Boeckel TP. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics. 2020;9(12):1–14. https://doi.org/10.3390/antibiotics9120918.
- 7.
Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health. 2008;29:151–69. https://doi.org/10.1146/annurev.publichealth.29.020907.090904.
- 8.
Choisy M, Van CN, Bao TD, Kiet BT, Hien BV, Thu HV, et al. Assessing antimicrobial misuse in small-scale chicken farms in Vietnam from an observational study. BMC Vet Res. 2019;15(1):206. https://doi.org/10.1186/s12917-019-1947-0.
- 9.
Swann MM, Blaxter KL, Field HI, Howie JW, Lucas IAM, Millar ELM, et al. Report to the joint committee on the use of antibiotics in animal husbandry and veterinary medicine. House of Commons Parliamentary Command Papers. 1969.
- 10.
Asai T, Kojima A, Harada K, Ishihara K, Takahashi T, Tamura Y. Correlation between the usage volume of veterinary therapeutic antimicrobials and resistance in Escherichia coli isolated from the feces of food-producing animals in Japan. Jpn J Infect Dis. 2005;58(6):369–72.
- 11.
Andersen VD, De Knegt LV, Munk P, Jensen MS, Agerso Y, Aarestrup FM, et al. The association between measurements of antimicrobial use and resistance in the faeces microbiota of finisher batches. Epidemiol Infect. 2017;145(13):2827–37. https://doi.org/10.1017/S0950268817001285.
- 12.
Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother. 2014;69(3):827–34. https://doi.org/10.1093/jac/dkt443.
- 13.
Alhaji NB, Haruna AE, Muhammad B, Lawan MK, Isola TO. Antimicrobials usage assessments in commercial poultry and local birds in north-Central Nigeria : associated pathways and factors for resistance emergence and spread. Prev Vet Med. 2018;154:139–47. https://doi.org/10.1016/j.prevetmed.2018.04.001.
- 14.
Kamini MG, Keutchatang FT, Mafo HY, Kansci G, Nama GM. Antimicrobial usage in the chicken farming in Yaoundé, Cameroon: a cross-sectional study. Int J Food Contam. 2016;3:10. https://doi.org/10.1186/s40550-016-0034-6.
- 15.
W H O Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR). Critically Important Antimicrobial for Human Medicine: Ranking of medically important antimicrobials for risk management of antimicrobial resistance due to non-human use. 6th ed. Geneva: World Health Organization; 2018.
- 16.
Jibril AH, Okeke IN, Dalsgaard A, Menéndez VG, Olsen JE. Genomic analysis of antimicrobial resistance and resistance plasmids in salmonella serovars from poultry in Nigeria. Antibiotics. 2021;10(2):1–22. https://doi.org/10.3390/antibiotics10020099.
- 17.
Amaechi N. A survey on antibiotic usage in pigs and poultry birds in Abia state. Nigeria Streptomycin. 2014;3(3):38–43.
- 18.
Græsbøll K, Damborg P, Mellerup A, Herrero-Fresno A, Larsen I, Holm A, et al. Effect of tetracycline dose and treatment mode on selection of resistant coliform bacteria in nursery pigs. Appl Environ Microbiol. 2017;83(12):e00538–17. https://doi.org/10.1128/AEM.00538-17.
- 19.
Al-Mustapha AI, Adetunji VO, Heikinheimo A. Risk perceptions of antibiotic usage and resistance: a cross-sectional survey of poultry farmers in Kwara state, Nigeria. Antibiotics. 2020;9(7):378. https://doi.org/antibiotics9070378.
- 20.
Joshua A, Moses A, Ezekiel OA. A Survey of Antimicrobial Agents Usage in Poultry Farms and Antibiotic Resistance in Escherichia coli and Staphylococci Isolates from the Poultry in Ile-Ife, Nigeria. J Infect Dis Epidemiol. 2018;4(1):4–11. https://doi.org/10.23937/2474-3658/1510047.
- 21.
Joosten P, Sarrazin S, Van Gompel L, Luiken REC, Mevius DJ, Wagenaar JA, et al. Quantitative and qualitative analysis of antimicrobial usage at farm and flock level on 181 broiler farms in nine European countries. J Antimicrob Chemother. 2019;74(3):798–806. https://doi.org/10.1093/jac/dky498.
- 22.
Rybak MJ, McGrath BJ. Combination antimicrobial therapy for bacterial infections. Guidelines for the clinician Drugs. 1996;52(3):390–405. https://doi.org/10.2165/00003495-199652030-00005.
- 23.
Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25(3):450–70. https://doi.org/10.1128/CMR.05041-11.
- 24.
Vestergaard M, Paulander W, Marvig RL, Clasen J, Jochumsen N, Molin S, et al. Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int J Antimicrob Agents. 2016;47(1):48–55. https://doi.org/10.1016/j.ijantimicag.2015.09.014.
- 25.
National Agency for Food and Drug Administration and Control (NAFDAC). Veterinary Medicine and Allied Products (VMAP), vol. 3; 2017. Available from: https://www.nafdac.gov.ng/about-nafdac/nafdac-organisation/directorates/veterinary-medicine-and-allied-products/
- 26.
Carrique-mas JJ, Trung NV, Hoa NT, Mai HH, Thanh TH, Campbell I, et al. Antimicrobial Usage in Chicken Production in the Mekong Delta of Vietnam. Zoonoses Public Health. 2015;62:70–8. https://doi.org/10.1111/zph.12165.
- 27.
Guetiya Wadoum RE, Zambou NF, Anyangwe FF, Njimou JR, Coman MM, Verdenelli MC, et al. Abusive use of antibiotics in poultry farming in Cameroon and the public health implications. Br Poult Sci. 2016;57(4):483–93. https://doi.org/10.1080/00071668.2016.1180668.
- 28.
Bashahungm D, Odocha T. Assessment of antibiotic usage in intensive poultry farms in Wakiso District, Uganda. Livest Res Rural Dev. 2015;27:12 Retrieved May 7, 2021, from http://www.lrrd.org/lrrd27/12/bash27247.html.
- 29.
Oluwasile B, Agbaje M, Ojo O, Dipeolu M. Antibiotic usage pattern in selected poultry farms in Ogun state. Sokoto J Vet Sci. 2014;12:1. https://doi.org/10.4314/sokjvs.v12i1.7.
- 30.
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A. 2015;112(18):5649–54. https://doi.org/10.1073/pnas.1503141112.
- 31.
Scott AM, Paula JF-C. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34(3):S93–S106. https://doi.org/10.1086/340246.
- 32.
Filippitzi ME, Callens B, Pardon B, Persoons D, Dewulf J. Antimicrobial use in pigs, broilers and veal calves in Belgium. Vlaams Diergeneeskd Tijdschr. 2014;83:1.
- 33.
Agunos A, Gow SP, Leger DF, Carson CA, Deckert AE, Bosman AL, et al. Antimicrobial use and antimicrobial resistance indicators – integration of farm-level surveillance data from broiler chickens and turkeys in British Columbia. Canada Front Vet Sci. 2019;6:131. https://doi.org/10.3389/fvets.2019.00131.
- 34.
Anonimous. Antimicrobial use in poultry 2013–2017 report. US Poult Egg Assoc. 2019;1:1–6 Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781118675014.ch34.
- 35.
Hog BB, Bager F, Korsgaard H, Ellis-Iversen J, Pedersen K, Jensen LB, et al. DANMAP 2017-use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. DANMAP. 2017;2018.
- 36.
Nielsen O, Sönksen W, Pedersen S, Lund P, Weber R. General rights Tackling antimicrobial use and resistance in pig production. Lessons learned in Denmark. Downloaded from orbit.dtu.dk on. 2019.
- 37.
Adene DF, Oguntade A. Poultry sector review: Nigeria. Food Agric Organ United Nations. 2008;1:1–95.
- 38.
Nigeria Centre for Disease Control (NCDC). National Action Plan for Antimicrobial Resistance 2017–2022. Federal Ministries of Agric Environ Health. 2007;136:1.
- 39.
Harada K, Asai T. Role of antimicrobial selective pressure and secondary factors on antimicrobial resistance prevalence in escherichia coli from food-producing animals in Japan. J Biomed Biotechnol. 2010;2010:180682. https://doi.org/10.1155/2010/180682.
- 40.
Nguyen NT, Nguyen HM, Nguyen CV, Nguyen TV, Nguyen MT, Thai HQ, et al. Use of Colistin and Other Critical Antimicrobials on Pig and Chicken Farms in Southern Vietnam and Its Association with Resistance in Commensal Escherichia coli Bacteria. Appl Environ Microbiol. 2016;82(13):3727–35. https://doi.org/10.1128/AEM.00337-16.
- 41.
Jibril AH, Okeke IO, Dalsgaard A, Kudirkiene E, Akinlabi OC, Bello MB, et al. Prevalence and risk factors of Salmonella in commercial poultry farms in Nigeria. PLoS One. 2020;15(9):e0238190. https://doi.org/10.1371/journal.pone.0238190.
- 42.
Thrusfield M, Brown H. Surveys. In: Veterinary Epidemiology. 4th ed; 2017.
- 43.
World Health Organization. Guidelines for and DDD assignment: WHO collaborating centre; 2013. p. 1–284.
- 44.
Team RC. R: a language and environment for statistical computing. Vienna; 2019.
Acknowledgements
We are most grateful to all managers and consulting farm veterinarians that assisted in recording antimicrobial usage in data sheets, as well as to staff affiliated to the Central Research Laboratory of Usmanu Danfodiyo University, Sokoto. We also acknowledge the effort of Mallam AbdulMalik Shuaibu for his technical assistance and to Dr. AbdulGaniyu Yusuf, Dr. Abubakar Umar Bunza and Mallam Faruk Umar for their support during data collection in the field.
Funding
An African Research Leader Award to INO from the UK Medical Research Council (MRC) and the UK Department supports INO for International Development (DFID) under the MRC/DFID Concordat agreement that is also part of the European Union EDCTP2 programme. The funder had no role in the implementation of the study.
Ethics declarations
Ethics approval and consent to participate
The Ethical approval to conduct study was obtained from Ministries and Directorate in Sokoto, Kebbi and Zamfara with approval reference numbers MAH&FD/VET/166/11, MAHF/VET/VOL1, and DAHLD/SUB/VET/VOL.1 respectively. Written consent to participate in the study was obtained from farmers.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interest to declare
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.