Source: PoultryHealthToday.com
Vigorous research and the application of cutting-edge technology by Zoetis scientists were behind the development of the newest recombinant vaccine to protect broilers from Newcastle and Marek’s disease viruses.
The vaccine, Poulvac® Procerta™ HVT-ND, was years in the making, explained molecular biologist and microbiologist Sing Rong, PhD, project team leader and research director, Zoetis.
Herpesvirus of turkey (HVT) is an avirulent virus that will replicate in chickens. It’s long been known that HVT is not only an effective vaccine for Marek’s disease (MD) in chickens, it can serve as a vector for delivering avian antigens that protect against other diseases. Vectored vaccines do not interfere with maternal antibodies, and they eliminate the reactions that can occur with live viral vaccines.
In Poulvac Procerta HVT-ND, the protective antigen is the Newcastle disease (ND) virus fusion (F) protein, which is vectored by HVT. Use of the F protein in the vaccine isn’t unique, Rong said, but what is worth noting is the rigor the team applied to the vaccine’s construction and candidate selection.
“We had stringent criteria for selecting a recombinant before we finally decided we had a winner based on its excellent efficacy, a 19-day onset of immunity against virulent ND virus as well as its stability,” she said.
The Zoetis team constructed and tested 13 different recombinants using the latest molecular techniques available. They identified the best site for insertion of the F protein-expression cassette into the HVT genome.
“In scientific terms, this involved testing various combinations of promoters, antigen and poly A sequences and inserting target gene-expression cassettes at various sites of the HVT genome. It’s the combination of elements rather than a single critical element that we credit with the vaccine’s highly effective immune response and the protection it provides,” Rong said.
Efficacy results
The team evaluated the vaccine’s efficacy in a study with specific-pathogen-free (SPF) birds. The vaccine was administered either in ovo or by subcutaneous injection at day of hatch, then birds were challenged with a virulent — also known as a velogenic — ND virus on day 28. Poulvac Procerta HVT-ND provided 93% protection in the in ovo group and 100% protection in the subcutaneous group. That study also confirmed the vaccine’s effectiveness against a virulent MD virus challenge.1
In commercial broilers, the vaccine’s efficacy was evaluated after in ovo administration, which is widely used by large poultry companies. When the broilers were challenged on day 33 of age with a velogenic ND virus, 100% were protected compared to only 8% of broilers that were unvaccinated and challenged,2 Rong said.
Onset of immunity
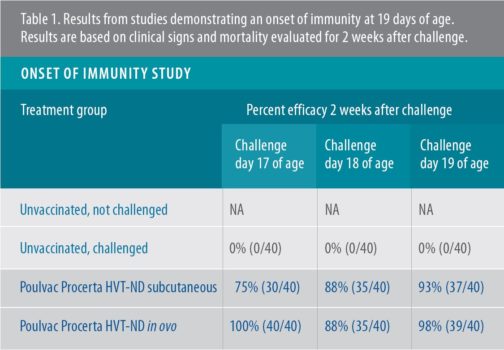
The 19-day onset of immunity with Poulvac Procerta HVT-ND was demonstrated in a controlled study with SPF birds. The vaccine was administered either in ovo or by subcutaneous injection at the minimum effective dose. Birds were subsequently challenged on 17, 18 or 19 days of age with a velogenic ND virus, then were followed for 2 weeks after vaccination for clinical signs and mortality.
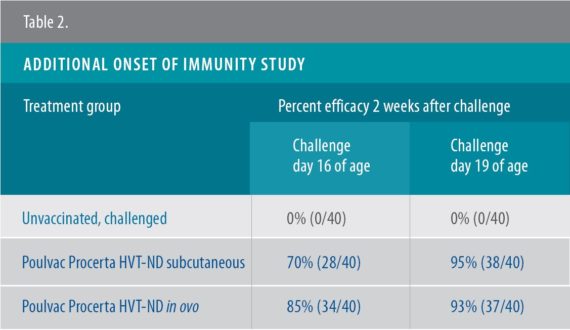
According to Rong, Poulvac Procerta HVT-ND conveyed full protection — defined as 90% protection or greater — by 19 days of age.3 Those results were then confirmed in an additional study3a, Rong said (Tables 1, 2).
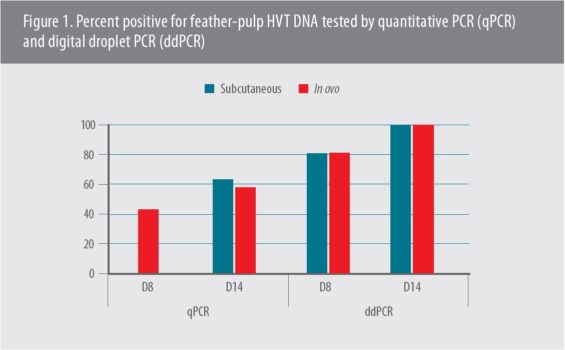
The team demonstrated vaccine uptake by isolating HVT-ND viral DNA from feather pulp and analyzed it by two methods: quantitative real-time polymerase chain reaction (qPCR) assays and digital droplet PCR (ddPCR) (Figure 1),4 she said, and noted the feather-pulp ddPCR test provides a way to determine if the vaccine has been administered correctly.5
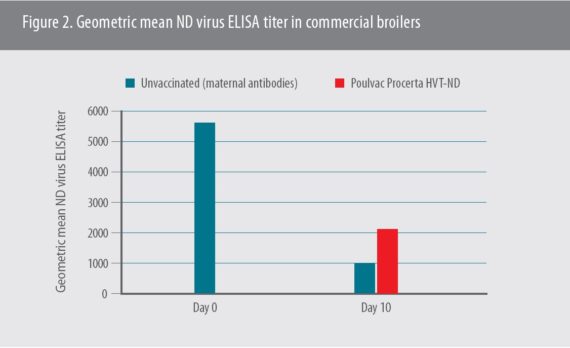
Although the onset of immunity was found to be 19 days of age, another study in commercial broilers showed there was a clear increase in the geometric mean antibody response to the vaccine as early as 10 days of age based on ELISA results,6 Rong said (Figure 2).
The vaccine’s high level of safety when administered by either route was demonstrated in field trials conducted in Arkansas, Georgia and Alabama. “Compared to controls, there were no significant differences in the percent of eggs hatched nor in broiler bodyweight or percent mortality or condemnations,”7 she said.
Compatible with Poulvac Bursaplex®
US poultry producers are acutely aware that the ND virus is an extremely contagious respiratory disease that can result in mortality, she continued. MD causes immune suppression that predisposes broilers to secondary infections, and it’s an important cause of condemnations.
“However, another major concern is infectious bursal disease (IBD), which prompted us to make sure the new vectored HVT-ND vaccine can be administered with Poulvac Bursaplex®, a classic, live viral vaccine with complexed antibodies,” Rong said.
In two separate studies, SPF leghorns received Poulvac Procerta HVT-ND along with Poulvac Bursaplex, either in ovo or by subcutaneous injection on day of hatch, and were challenged with a velogenic strain of ND or with a virulent classic IBD virus.
Based on parameters including efficacy, serology, mortality and clinical observation, the vaccines were found to be compatible when administered together,8 she said.
As the use of antibiotics in broilers has declined, the importance of vaccination to establish immunity against viruses has increased because viruses not only make chickens sick, they predispose them to secondary bacterial infections, Rong emphasized.
“Poulvac Procerta HVT-ND provides a way to help flocks develop foundational immunity against ND without the reactions that can occur with live vaccines. That means less stress on birds, which is especially important when birds also need vaccination with live vaccines for protection against other diseases like infectious bronchitis as well as IBD,” she said.
1 Luo Y, et al. Construction and efficacy of a recombinant HVT-ND vaccine against NDV and MDV challenges in SPF and NDV challenge in broiler birds. Am Associat Avian Pathol. 2019.
2 Ibid.
3 Data on file. Study No. B815R-US-18-A46. Zoetis LLC.
3a Data on file. Study No. B815W-US-18-964. Zoetis LLC.
4 Bosserd M, et al. Onset of immunity of a recombinant HVT-ND against a velogenic NDV challenge in SPF birds. Am Associat Avian Pathol. 2019.
5 Dimitrov K, et al. Newcastle disease vaccines — A solved problem or a continuing challenge? Vet Microbiol 2017;206:126-136.
6 Data on file. Study No. B810W-US-16-700. Zoetis LLC
7 Data on file. Study Nos. B911R-US-18-902, B911R-US-18-903, B911R-US-18-904 and B911R-US-18-906. Zoetis LLC.
8 Turner-Alston K, et al. Compatibility of a Recombinant HVT-ND Vaccine with Bursaplex to Provide Protection against Velogenic NDV and Virulent Classic IBDV Challenge in SPF Birds. Am Associat Avian Pathol. 2019.
DISCOVERIES, Issue 5
Discoveries is a series of research news reports written by the editors of Poultry Health Today on behalf of the US Poultry Business of Zoetis.
BIO-00221
May 2020