Broiler Breeder Management How-to’s provided by Aviagen
Tip #7
Chain feeder speed determines how quickly feed is distributed.
Broiler Breeder Management How-to’s provided by Aviagen
Tip #7
Chain feeder speed determines how quickly feed is distributed.
Source: USDA news release
To view the complete report, click here.
The Livestock, Dairy, and Poultry Outlook for February 2020 analyzes economic impacts on animal product markets of month-to-month changes in USDA’s World Agricultural Supply and Use Estimates Report.
On February 3, 2020, an error was corrected in a graph on U.S. broiler exports to China, published in the December 2019 Livestock, Dairy, and Poultry Outlook report. The original graph underrepresented U.S. broiler exports to China, although the corresponding written analysis was accurate.
New research exploring the links between animal nutrition and health is offering promise and generating much excitement in helping solve some of today’s greatest livestock production challenges. Participants from across the feed industry will have the opportunity to learn about the latest trends and scientific developments in this area, at the annual Animal Nutrition Conference of Canada (ANCC), being held on May 12-14 in Winnipeg, Manitoba.
“The feed industry is evolving at a rapid pace to meet new regulatory and customer requirements,” says Melissa Dumont, Executive Director of the Animal Nutrition Association of Canada (ANAC), host of this conference. “The ANCC has always focused on the latest scientific developments in animal nutrition to ensure participants are ready for our industry’s changes. Add to that the opportunity to network with a broad spectrum of industry leaders in the field of animal nutrition and the result is this exceptional event.”
The 2020 ANCC theme “Exploring the Links Between Animal Nutrition and Animal Health” tackles one of the hottest topics in our industry today, says Amy Johnston of Manitoba Agriculture, ANCC Program Chair. “It’s a theme that many can relate to, as we investigate alternative antimicrobial approaches, focus on food safety and promote sustainable agricultural practices. There is increasing interest in the role that animal nutrition can play in supporting animal health.”
Now in its fourth year, the ANCC brings together members of the feed industry into one national event featuring top speakers, engaging topics and the latest scientific information, along with excellent networking opportunities and a special focus on highlighting and supporting student research. A balance of plenary sessions with broad appeal and species-specific technical sessions, both for monogastrics and ruminants, can be found at the conference. The research showcased at ANCC provides a unique window on the future and is testimony to the active animal science research happening across Canada and around the world. The event also includes an industry showcase featuring partners from across the animal nutrition supply chain as well as a graduate student poster competition.
“We are very pleased to welcome the ANCC to Winnipeg in 2020,” says Rhett Arnason of Pestell Nutrition, based in Manitoba and serving on the ANAC Executive Committee. “Each year brings a new opportunity to highlight a different region important to our dynamic Canadian feed industry and to bring people together from across the country. There is strong benefit in connecting and engaging nationally, for the participants and sponsors alike.”
For nutritionists, ANCC has become a must-attend event, says Jennifer Lichty of Hensall District Co-op based in Ontario, who serves as ANAC Nutrition Committee Chair. “For many of us, it’s the one time each year we get together as a community of animal nutritionists to focus on issues that affect our industry as a whole. The opportunity to share diverse knowledge and discuss possible solutions to our common and unique issues is quite beneficial – particularly with the growing focus on nutrition as a tool to manage challenges and costs. There is a lot of synergy created when we bring everyone together.”
Early registration rates are available until March 23rd. Complete program details, ongoing sponsorship opportunities and registration information are available at www.animalnutritionconference.ca. Learn more about ANAC at www.anacan.org.
Lambertson Family Farms of Pocomoke, Maryland, was one of six farms across the United States to receive U.S. Poultry & Egg Association’s Family Farm Environmental Excellence Award during the 2020 International Production & Processing Expo in Atlanta. U.S. Poultry & Egg Association (USPOULTRY) sponsors the annual awards in recognition of exemplary environmental stewardship by family farmers engaged in poultry and egg production.
Applicants were rated in several categories, including manure management, nutrient management planning, community involvement, wildlife enhancement techniques, innovative nutrient management techniques, and participation in education and outreach programs. Applications were reviewed and farm visits conducted by a team of environmental professionals from universities, regulatory agencies and state poultry associations.
Lambertson Family Farms has been located on the Eastern Shore of Maryland in the Chesapeake Bay Watershed for generations. The family raises broiler chickens for Tyson Foods.
In such an environmentally sensitive area, the issue of resource management and responsible farming is crucial, and the Lambertson family has made sustainability a key facet of their operation. In a testament to this dedication, the Lambertsons have been voluntarily implementing a nutrition management program at their farm since 1995, long before it was mandated by the state of Maryland. Throughout the years, their conservation efforts have expanded to include the creation of vegetative buffers and wildlife habitats, as well as a consistent system of crop rotation and no-till farming to preserve the soil’s nutrients. Any manure from their poultry operation is carefully transferred from poultry house to field, strictly adhering to the standards of the Maryland Manure Transport Program.
The Lambertsons have made a point of making sure their farms are a part of the innovative future of agriculture. By partnering with and providing land to a local company, the Lambertson Family Farms have become a testing ground for innovative new technologies in anaerobic digestion in manure management, a process which mitigates the levels of phosphorus in the final product and creates green energy for use on the farm. They have also implemented solar panels throughout the farm, which has provided their operation with a cheaper and more sustainable source of energy. These tech-savvy and sustainable systems have made Lambertson Family Farms a source of interest in their community, which they have encouraged by opening their farm to educational tours and visits from those interested in the agricultural industry.
The Lambertsons are dedicated to protecting the environment in which they and their families work and live. The Chesapeake Bay Watershed, upon which they and their communities depend on, is such a sensitive yet vital resource. The Lambertsons view it as their responsibility to make sure that they are good stewards of the environment that has provided for their families.
Mike Levengood, vice president, Chief Animal Care Officer and Farmer Relationship Advocate for Perdue Foodsand vice chairman of the USPOULTRY board of directors, presented the award to Wayne, Mary, Jason and Logan Lambertson.
The mess left behind by broken eggs is an obvious reason for you to care about the shell quality of the eggs laid by your hens. A less visible reason is the higher level of salmonella found in cracked compared to intact eggs – especially if they are improperly washed.
The most common causes of weak shells eggs in your flock are:
Less common problems include insufficient manganese in the feed, toxins or pesticides, and miscellaneous diseases such as coccidiosis and Infectious Laryngotracheitis (ILT).
Shell quality can be improved by providing the nutrients needed for the hen to build her bone reserves of calcium and make good shells:
These practices will not stop the natural weakening of the shells as your hens age but will help to prevent any premature problems. If your old hens go into a moult, shell thickness will be temporarily improved.

Weak Shells can lead to cracks & “Leakers”/ Photo- University of Georgia Cooperative Extension Service
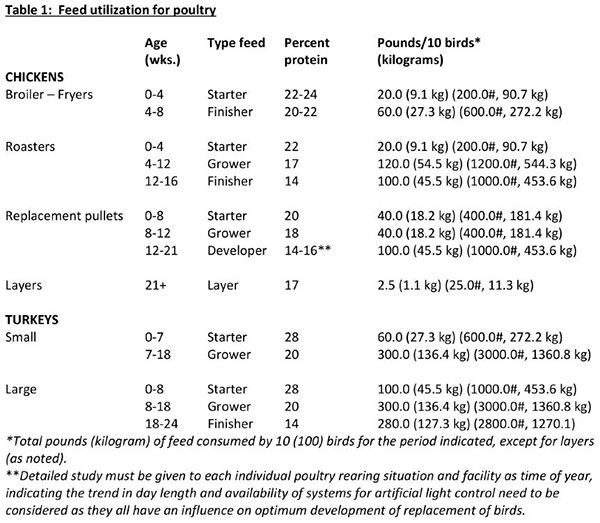
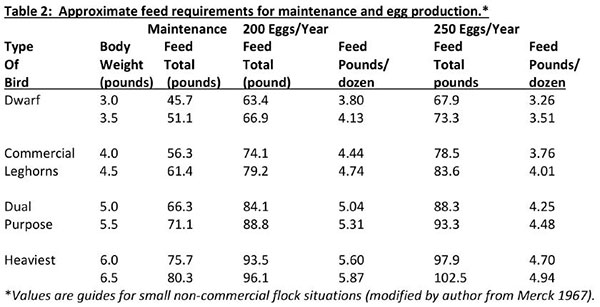
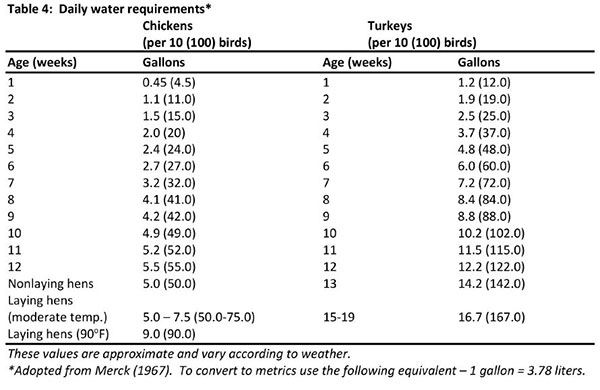
It is important to maintain health and productivity of poultry through proper nutrition. Birds need a series of nutrients which are found in the various feed ingredients. These nutrients include macronutrients (proteins, carbohydrates and fats), micronutrients (minerals and vitamins) and water. Water is not usually considered as a nutrient, but its importance must always be emphasized. There are several systems of feeding: free-choice or “cafeteria style” feeding of mash and grain, controlled feeding of mash and grain, feeding all mash, or other combinations of a complete feed.
Each system should accommodate the specific needs of your flock, and be designed for flexibility, low maintenance, and reliability to keep installation and operating costs low. The choice of one of these feeding systems will depend mainly upon the size of the flock and the labor and equipment available. Success with any system depends on the feed supply, equipment, management and individual practices. The likelihood of disease and/or nutritional problems will be minimized if good sanitation, adequate housing, equipment and daily care are emphasized. Regardless of the quality of chicks purchased, good results cannot be expected unless chicks are fed a nutritious diet. Free choice allows birds to balance or regulate their intake of grain and mash. The freechoice system can work well with small flocks but leaves too much guesswork for a commercial flock. There are, however, general recommendations for feeding replacement chicks, layers, broilers and turkeys.
Controlled mash and grain feeding is used successfully in small flocks. More care and attention must be given to the flock fed this way than the flock fed with all-mash. Controlled mash and grain feeding involves the use of a concentrate (20 to 23 percent protein), and limited amounts of whole grains and calcium supplement. The amount of grain fed should be calculated to make the total feed intake contain about 16 percent protein. Slight adjustments can be made seasonally to provide more energy in cold weather (more grain) and to provide less energy in hot weather (less grain). Grains may be placed in hoppers or scattered in the litter.
The all-mash system is by far the most common. This is especially true for commercial size flocks. The proper nutritional balance is taken care of by man rather than letting the hen balance her own ration. In larger flocks this ensures against the possibility that the hen will not do a good job of balancing her own ration. All-mash feeding involves the use of a single mash which contains all components of a balanced ration. Egg quality actors, such as shell thickness and yolk color, are more uniform and more easily controlled with this system. An all-mash ration can be easily dispensed in hanging or automatic feeders. Less skill on the part of the flock owner is required with the all-mash system. A calcium supplement such as crushed oyster shell or limestone granules can be made available freechoice, if desired. Also, a portion (1 / 2 or 2/ 3) of the calcium supplement in the ration may be larger particle size limestone or oyster shell. Calcium supplements that are larger take longer to move through the digestive system and therefore more calcium is absorbed. Small particle size supplements move quickly through the digestive system and less calcium is released into the bloodstream.
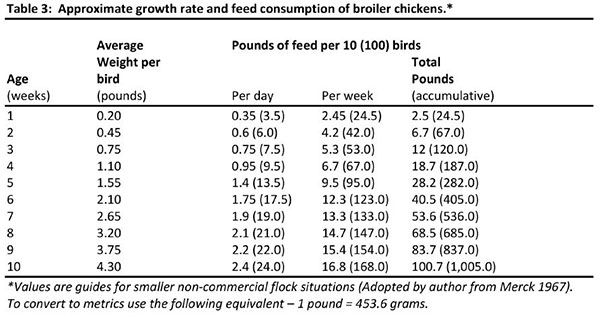
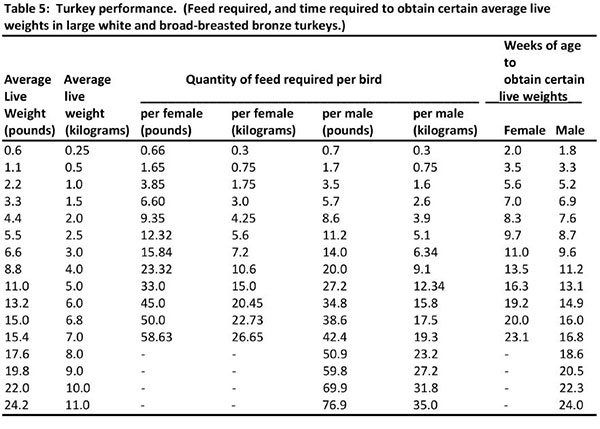
A complete ration purchased from a local feed supplier will be the most easily managed, and the likelihood of disease risk and/or nutritional problems will be minimized if good sanitation, adequate housing, equipment and daily care are emphasized. The actual feed requirement for any group or flock will vary depending upon many factors. However, the figures listed below can be followed as the best estimate of feed utilizations. Weights are based on 10 birds with weights for 100 birds in parenthesis.
When using a commercial feed, the manufacturer’s feeding recommendations should be followed explicitly. Avoid mixing recommendations of several different companies because each manufacturer designs their product for a specific feeding program.
Feed form – either mash, crumbles or pellets – are other considerations and each has its specific advantage and disadvantage and varies for optimum utility among the various ages and poultry species. In short, there is not one best answer to a correct choice: however, mash is generally the most economical feed store purchase.
No one feeding system is the best – the choice is an individual one. Generally, commercial producers follow the all-mash system, while families with backyard poultry enterprises and hobby poultry producers generally follow either the free choice or controlled mach and grain feeding system.
The new CFIA Humane Transportation Regulations for livestock has now come into affect. For broiler chickens and spent hens the maximum interval without water is now 24 hours, and 28 hours without feed. For hatching birds (chicks only) the regulation has not changed; the maximum interval without feed and water is 72 hours from time of hatching. To download the full Transportation Fact Sheet, click the link below.
Coccidiosis is a common disease of poultry caused by protozoan-type parasites (coccidia). Theseparasites live and multiply in the intestinal tract and cause tissue damage. The resulting damage interferes with food digestion and nutrient absorption. Dehydration and blood loss are possible aswell. Also, the tissue damage can make the affected bird susceptible to infection by bacteria, such asClostridia and Salmonella.
Birds infected with coccidia may shed oocysts in their feces for days or weeks. The oocysts transforminto spores in litter, soil, feed, or water. Susceptible birds in the same flock then ingest the sporulated oocysts and become infected. Infected feces or litter can contaminate boots, clothing, and equipmentand can be spread to additional flocks. Coccidiosis is prevented through good sanitation and litter management. An important aspect of litter management is elimination of wet litter, which isespecially prevalent under waterers. Coccidia are resistant to harsh environmental conditions and common disinfectants. It is important to change litter of highly infested flocks. Use of anticoccidial medications or vaccines is never a substitute for good management practices.
Anticoccidial medications can be used in conventional poultry production (not organic poultry production) and commonly are added to poultry feed to prevent coccidiosis. Anticoccidial medications used in poultry feeds include the following examples:
amprolium (e.g., Amprol, Corid)decoquinate (e.g., Deccox)diclazuril (e.g., Clinacox)halofuginone hydrobromide (e.g., Stenorol)lasalocid (e.g., Avatec)monensin (e.g., Coban)narasin (e.g., Monteban)nicarbazin (e.g., Nicarb 25%)salinomycin (e.g., Bio-Cox, Sacox)semduramicin (e.g., Aviax)sulfadimethoxine and ormetoprim 5:3 (e.g., Rofenaid
Because anticoccidial medications may not be used in organic poultry production, alternative meansof control must be used. One option is the use of coccidiosis vaccination. It is important to understand the differences among coccidiosis vaccines. First, different coccidiosis vaccines exist for different poultry species because different strains of coccidia attack different species. For example,some coccidia affect chickens but not turkeys, and others affect turkeys but not chickens. Second,there are two types of coccidiosis vaccines: virulent and attenuated. Most coccidiosis vaccines arevirulent, meaning that they include a low dose of the live parasite as a key component for stimulating protective immunity. Attenuated vaccines lack a part of the life cycle of the original strain they werederived from. As a consequence, they have a lower productive and pathogenic potential. This characteristic is a major advantage of attenuated vaccines over virulent vaccines.
Coccidiosis vaccination is not infallible. Heavy infestations of coccidia can cause disease even in vaccinated chickens if the chickens’ immune systems are compromised, damaged, or suppressed by other infectious agents.
Coccidiosis vaccines include the following options:
-Coccivac®-B—live oocysts of chicken isolates
-Coccivac®T—live oocysts of turkey isolates
-Paracox®-5—live attenuated vaccine for chickens
NOTE Brand names appearing in this article are examples only. No endorsement is intended; nor iscriticism implied of similar products not mentioned.
NOTE Before using any product, make sure that the brand name product is listed in your Organic
System Plan and approved by your certifier.
Source: PoultryHealthToday.com
Avian mycoplasmosis is a problem for poultry producers worldwide, but according to an expert, there’s no “one-size-fits-all” approach to managing it.
Speaking to Poultry Health Today at the 2019 World Veterinary Poultry Association Congress in Bangkok, Naola Ferguson-Noel, DVM, MAM, PhD — a professor at the University of Georgia — said the most effective interventions and control methods for mycoplasma largely depend on where you are, which segment of the industry you work in and what your goals are.
In the US, for example, she said the focus has been on mycoplasma eradication, with particularly successful results in the broiler breeder industry. However, she acknowledged, elimination is not always a viable strategy in other countries where the prevalence of mycoplasma is much higher.
“With the eradication approach, you need to have a pretty low incidence of disease and what you do is…eliminate anything that is positive, so that it is not a source of infection for the rest of the industry. If you have a very high prevalence, you can’t do that,” Ferguson-Noel explained.
If elimination isn’t an option, vaccines and medication may be used, but it’s important to know which interventions are likely to be most effective, she stressed.
“Vaccines and medications are all slightly different. They’re not all equally safe, and they’re not all equally efficacious. So there has to be some thought in terms of which vaccine to use in which particular situation.”
According to Ferguson-Noel, the key to an effective mycoplasma-control plan lies in diagnostics.
“Diagnostics are very important to coming up with an effective plan and making sure that your plan is working. And if it’s not, you can make changes,” she said.
“So [if] you’re trying to use one vaccine and you find out that it’s not efficacious enough for your situation…you switch to another vaccine, or you add a second vaccination.”
Mycoplasma diagnostics have been available for decades, Ferguson-Noel said, ranging from the relatively “low-tech” serum plate agglutination test to more sophisticated polymerase chain reaction (PCR) testing. In recent years, real-time and strain-specific PCR tests have enabled the distinction between vaccine strains and field strains, allowing producers to more accurately assess the mycoplasma situations on their farms.
Looking at the future of mycoplasma diagnostics, Ferguson-Noel said next-generation sequencing technology — which can rapidly map out the entire genome of mycoplasma and other respiratory pathogens from a tracheal swab — is allowing producers to gather much more data more quickly.
“Because we’re looking at the entire genome, we have much more information than we would have with short pieces of DNA. So we are able to identify key factors that will be important to the veterinarians and the producers — things like whether there is antibiotic resistance,” she said.
“We will be looking for virulence factors, and we’re able to actually come up with actionable data for the practitioners.
“Hopefully, some of these new techniques will give us more information and fast information in terms of what is going on in the flocks — and give the veterinarians and the producers and the farmers more information so that they can better control mycoplasma in the long term.”
Mycotoxins are estimated to affect 25 percent of the world’s crops and can cost an estimated at $466 million USD, while production losses tied to mycotoxins approach $6 million USD annually (CAST, 2003). When mycotoxins are present in poultry feedstuffs, it can have negative health and production effects, ranging from acute to chronic diseases, altered growth patterns, reduced production and reproductive efficiency, and susceptibility to infectious diseases.
There are five major groups of mycotoxins:
Aflatoxins and OTA are considered storage mycotoxins because they are primarily produced after harvest, during the storage phase. These mycotoxins are less common due to improving storage practices.
Trichothecenes, FUM and ZON are considered field mycotoxins because they are primarily produced while crops are growing in the field. These are found in feedstuffs more frequently due to uncontrollable weather conditions.
Corn, bran, distillers’ dried grains with solubles and peanut meal are the most common mycotoxin-contaminated feedstuffs in the poultry diet. When crops are contaminated while growing in the field, it is likely that several mycotoxins will be present at the same time. For example, if you find DON in a corn sample, it is likely that you will also find ZON and FUM in the same sample because they are produced by the same genus — fusarium.
Mold can also be found in poultry litter if the litter gets wet and, in some situations, can produce mycotoxins. Mold can be present anywhere and can survive a wide temperature range from 23 to 122 degrees Fahrenheit (-5 to 50 degrees Celsius). High humidity also creates favorable conditions for mold growth.
Mycotoxins can lead to decreased feed intake, liver failure and gastrointestinal epithelial tissue damage. This will decrease nutrient absorption, reduce a bird’s ability to mount a robust inflammatory response to immune challenges, and decrease the amount of energy available for health maintenance, leaving birds more susceptible to disease.
In laying hens, mycotoxins decrease egg production and eggshell quality. The effects are the same in breeders. Since AF and DON can be transferred to the egg, it can impair the formation of blood vessels and cause early death for embryos. In broilers, mycotoxins decrease growth and reduce carcass quality by making birds more susceptible to bruising and bleeding in the muscle tissue.
Overall, mycotoxins in feed increase the trace mineral requirement of animals, particularly those anti-oxidative minerals such as Zn, Mn and Se. Aflatoxins, DON and FUM cause damage to intestinal epithelial tissue and may reduce the absorption of nutrients, including essential trace minerals which are required in increasing quantities during a mycotoxin challenge. Supplementation with Zn, Mn, Cu and Se is required to repair damage to cell membranes and to counteract the oxidative stress caused by mycotoxins.
Looking for visual signs of mycotoxins is an unreliable strategy. Sometimes you can see mycotoxins if your poultry feed is really moldy, but the presence of mold doesn’t necessarily guarantee the presence of mycotoxins. On the other hand, grains that are clean and free of mold could have mycotoxins present, even at high levels.
The only way to truly identify if mycotoxins are present, and at what level, is to take samples and send them to a lab for chemical analysis. Be sure to collect samples from 20 to 30 points throughout your poultry feed supply as there may be spots with high contamination levels, and some with no mycotoxin contamination at all.
If mycotoxins are detected in your feed ingredients, there are three potential approaches you can take:
Option 1 — Eliminate the contaminated feedstuff. This is the ideal option. The problem is producers and nutritionists are often not in a situation where eliminating the feedstuff is an option, and very often the producers and nutritionists cannot find clean feedstuff without any mycotoxin contamination.
Option 2 — Reduce the feeding rate of the contaminated feedstuff. This option allows the producer and nutritionist to blend the contaminated feed with non-contaminated feed to reduce the mycotoxin contamination to an acceptable level. Even at a very low amount, however, mycotoxins will have a negative impact on immunity and health of an animal, but this point is often overlooked.
Option 3 — Use a research-proven mycotoxin sequestering agent to decontaminate the feed. Producers and nutritionists can blend a mycotoxin sequestering agent into the contaminated feed. The mycotoxin sequestering agent will bind the mycotoxin to the agent, making it inert and preventing it from being absorbed by the animal. However, producers and nutritionists may still need to implement Option 2 as part of Option 3 for improved response.
The best way to control mycotoxins is to prevent them from contaminating the feedstuffs at the point of production. In some circumstances, drought or high levels of precipitation that cause fungal diseases cannot be avoided. However, management of the growing crop can help to reduce the levels of mycotoxins. Important considerations are management of the soil, crop rotations, variety selection, fungicide application and reducing soil contamination during storage.
While mycotoxin sequestering agents are effective in controlling the mycotoxins, some classes of mycotoxin sequestering agents may also bind trace minerals, rendering them unavailable for absorption and metabolism. One way to overcome this potential and unintended consequence is to feed performance trace minerals. Performance trace minerals, including Availa®Zn, Availa®Mn, Availa®Cu and Availa®ZMC feature a trace mineral bound to an essential amino acid in a unique manner, which maximizes stability and absorption. This trace-mineral-to-amino-acid bond will not be impacted by a mycotoxin sequestering agent, allowing the trace minerals to be better absorbed and metabolized by the animal.
To learn more about mycotoxins and mycotoxin sequestering agents, download the Effects of Mycotoxins and Mycotoxin Sequestering Agents on Mineral Nutrition research paper written by Zinpro.