Broiler Breeder Management How-to’s provided by Aviagen
Tip #1
Broiler Breeder Management How-to’s provided by Aviagen
Tip #1
Source: Hybrid Turkey’s
Did you know the same genetic strain or product, raised in two different environments can achieve different results in performance? This is known as the “genotype by environment” effect (GxE). Our balanced breeding program accounts for GxE in a number of different ways to ensure that our turkeys perform well in a commercial environment; however we are raising the bar even further by establishing a Pedigree Complex in Europe.
This new Pedigree complex will be added to the existing one in Canada. By studying and making selections, in two separate geographic areas, we can offer products even further tailored to the production systems and environments of our customers around the world.
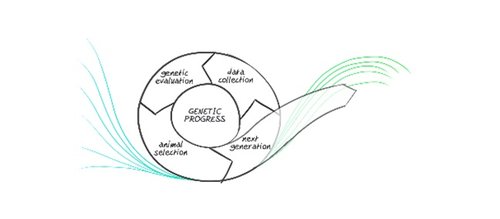 Turkey breeding is a complex process that requires time to implement changes. Our geneticists are generally thinking in terms of what the market will need 3-5 years from now, as that is how long it can take to see the effects of a breeding change at the commercial level of the value chain.
Turkey breeding is a complex process that requires time to implement changes. Our geneticists are generally thinking in terms of what the market will need 3-5 years from now, as that is how long it can take to see the effects of a breeding change at the commercial level of the value chain.
By establishing a pedigree facility in Europe we are able to select breeder candidates raised in similar conditions as their future offspring. In doing this we can speed up adjustments in the breeding program and adapt to changes in the market even faster than we do now. In addition, we will be able to compare data of animals in the same family from breeding through distribution, in local growing conditions, which provides a more robust product offering.
Our pedigree facilities will raise not only our core turkey strains, but also our coloured turkeys that are designed to address the needs of alternative and traditional market sectors
Our new pedigree complex will feature leading biosecurity measures to safeguard the turkeys of the future. All barns on the pedigree complex will require employees to shower-in and change clothes. This procedure ensures all potential pathogens or bacteria are removed prior to entering each barn. Risk of cross contamination is removed since each barn functions like a stand alone facility.
 In addition to adding flexibility to meet changing market demands, we will have the capacity to monitor birds individually in order to speed up our economic efficiency. One example is through the use of specially designed feed stations that will allow us to analyse the feed conversion of each animal and select the most efficient birds for the breeding program.
In addition to adding flexibility to meet changing market demands, we will have the capacity to monitor birds individually in order to speed up our economic efficiency. One example is through the use of specially designed feed stations that will allow us to analyse the feed conversion of each animal and select the most efficient birds for the breeding program.
The process to establish this Pedigree Complex is already in motion. First hatches to populate the facility began in April of this year. We anticipate the complex to be in full use by the end of the year.
We are proud to establish this complex in Europe as it remains a strong and important part of our business, both for Hybrid Turkeys and the other species within Hendrix Genetics. Our world-class team of research and development experts allow us to constantly improve our genetics to offer products the market demands. In addition to this, our support team remains available to ensure customers get the best possible results for their business.
Introduction
Keeping birds comfortable during hot, humid weather is critical for optimizing weight gains, feed conversion and livability. Improved growth rates and the trend to heavier average mar-ket weights contribute to greater heatloads in modern broiler barns. While the poultry industry has made significant strides to minimize seasonal effects, even the best housing design can still result in birds settling with lighter weights when nature turns up the temperature.
The Booming North America Poultry Processing Equipment Market and Opportunities for the Vendors:
Amidst the proliferating vegan wave in the North America countries wherein a myriad of people is believed to have turned towards the plant-based diet, it’s interesting to note that Animal Charity Evaluators conducted a survey that suggests that 2-6% of Americans self-identify themselves as vegetarians. While this may hold to be acting against the poultry industry, another revelation offers an oppositional reading. Out of all the Americans that claim to be vegetarians, 60% are still found to be consuming meat and poultry products. This is to say that despite the growth of a seemingly turning vegetarian population in America, the market for non-vegetarians products remains sustainable, and it includes poultry products that are ingredients of a variety of foods.
Owing to the consistent demand for poultry products in the region, North America held the maximum poultry processing equipment market share with 36% in 2018. The growth prospects for the poultry processing equipment market players in North America can be perceived by glancing through the scope for profitability in major markets of the US, Mexico, and Canada.
According to the National Chicken Council, per capita consumption (in pounds) of poultry products 94.7 in 2000 and the figure catapulted to 100 in 2018. This evinces the growth of the poultry industry in the US, which is increasing the US poultry processing equipment market size.
Furthermore, the latest 2012 Census of Agriculture had reported that there were 233,770 poultry farms in the US. With the increase in the demand for poultry products, this number has undergone an increment over the last 5 years. This increase in the end-user base in the US is bringing more opportunities on the table for the vendors.
According to the Organisation for Economic Co-operation and Development (OECD), the expansion in the consumption of chicken will continue in Mexico, and by the end of 2025, the demand will increase at a rate of 20%. While this will positively influence the local poultry farms, it will also create a massive impact on the US poultry industry, because Mexico imports a humongous amount of poultry products such as chicken, turkey, and broiler from the US. This increasing demand for chicken in Mexico will further expand the poultry processing equipment market scope in North America.
According to the findings by the government of Canada, the country houses 2,877 regulated chicken, 531 registered turkey, 243 broiler chicken, and 1,143 egg producers in 2018. Furthermore, Canada produced poultry and egg products worth $4.6 billion during the year. The egg and chicken production in massive quantity in various poultry farms creates a sustainable poultry processing equipment market demand in the country.
The sustainable demand for poultry processing equipment in the aforementioned countries resonates with the notably rising import of goods belongs to the parent industry.
Poultry Processing Equipment Market – Global Scenario & Growth Drivers:
The number of non-vegetarians in developing countries is growing continuously, especially due to the rise in disposable income, which is making the poultry products affordable to the residents. This is translating to the profitable growth of the poultry industry in APAC countries such as China, India, Indonesia, and others. For instance, the Food and Agricultural Organization (FAO), poultry production in India has been continuously rising at around 8% per annum with an average annual turnover of $7.5 billion in the last 2-3 decades. This evinces the growth of the poultry industry and an increase in the number of poultry farms which make up for a profitable poultry processing equipment marketplace for the vendors. The upcoming years foresee further development of the poultry industry in the developing countries, which will expand the poultry processing equipment market size.
The most lucrative application segment in the poultry processing equipment market is killing and de-feathering equipment, and it is poised to grow at a CAGR of 4.8% through to 2025. While the vendors will be venturing into this segment, it is important to note that the quality of plucking will hold utmost importance to the end-users or poultry farms.
One of the major objectives in the SDGs by the UN is to ameliorate the global nutritional deficiency, which will lead to an expansion of the food industry at large. The poultry industry will be experiencing collateral profitability during this development, which is going to offer opportunities to the associated markets that include poultry processing equipment market.
Poultry Processing Equipment Market – Competitive Landscape:
The major poultry processing equipment market players that are striving to capitalize on the rising requirement from the poultry farms are Marel, Key Technology Inc., John Bean Technologies Corporation, CTB Inc., BAADER Food Processing Machinery Inc., Brower Equipment Sales, Inc., BAYLE SA, CG Manufacturing and Distributing, Inc., Prime Equipment Group, Inc., and Brower Equipment.
Talk to one of our sales representative about the full report by providing your details in the link below:
https://www.industryarc.com/support.php?id=504480
Related Reports:
a) Processed Poultry and Meat Market
https://www.industryarc.com/Report/7493/processed-poultry-meat-market.html
b) Meat and Poultry Safety Testing Market
https://www.industryarc.com/Report/233/meat-poultry-safety-testing-market-report.html
Many factors can influence egg quality and appearance. These factors will vary depending upon the housing environment, genetics, drugs, feed ingredients, or chemicals used in agriculture. As egg production methods become more varied—for example, changing from cage to free range—and as layer strains are selected for the environment and hens age, the number of defects in eggs is increasing. The emphasis on locally produced food in the “slow food movement” also has changed what the hens are exposed to and made the way they are handled increasingly variable. It is important for producers to understand what may cause certain egg defects and to provide a solution in their production system.
The following chart identifies a number of defects in eggs, their possible causes, and corrective measures you may take.
| Shell | ||
| Defect | Cause | Corrective Measure |
Abnormal shell color:
|
Nicarbazine | Do not allow in hen area or in feed. |
| Chlortetracycline (600-800 gm/ton), Aureomycin | Use dosages recommended for treatment. | |
| Exposure to gas leaks (natural gas or propane) from heaters | Maintain gas lines and equipment in proper working order; gas leaks are an explosion hazard as well. | |
| Long-term stress | Minimize hen stress in the production system. Watch out for problems with density, nutrition, environment control/temperature, noxious gases. | |
| Respiratory disease; infectious bronchitis (IBV) | Follow the recommended vaccination program during rearing; include periodic booster vaccinations. | |
| Excessive calcium in the feed (causing brown shell eggs to be off-color) | Ensure that the diet formulation contains the correct amount of calcium according to the Nutrient Requirements of Poultry, 1994, 9th edition, National Research Council. | |
| Mottling of shell (bright spots or moist appearance around pores, observed by candling) | Failure of the egg cuticle on the shell to dry quickly after laying. High humidity in the production house or cooler As a result, water is retained by protein in the spongy later of the shell. |
Lower humidity in the egg-holding cooler below 80% RH. Humidity can be difficult to lower in hen houses, but cuticle drying may be accelerated with increased ventilation. |
| Disease – infectious bursal disease (Gumburo) | Make sure that layer stock come from parent stock vaccinated against IBD (Gumburo). | |
| Crowding | Avoid overstocking. | |
| Abnormal shell colors due to egg washing: white to brown | Iron (FeSo47H2O) above 1 ppm | Have iron content checked in well water used for washing eggs; keep below 1 ppm. |
| Faded color | Low calcium or crab shell in layer diets | Raise calcium level for increased shell color and improved shell thickness. |
|
Arasan (tetramethylthiuram disulfide) | Do not feed to allow hens access to grains treated with Arasan; law requires treated seeds to be dyed. |
| Sulfanilamide (sulfa drugs) | Use according to directions or veterinary prescription. | |
| High hen house temperatures | Control the temperature. Provide cool water and air movement. | |
| Respiratory diseases: Newcastle disease, infectious bronchitis (IBV), laryngotrachetis (LT), Mycoplasma gallisepticum (MG), and egg drop syndrome | Follow the recommended vaccination program during rearing; include periodic booster vaccinations. | |
| High salt (NaCl) | Feed less salt and/or reduce other mineral salts in the diet. Changes are dependent on current levels and regional needs. | |
| Drugs used for rodent control | Keep rodenticides away from poultry. | |
| Age of hen | Eggs can be misshapen from young hens with an immature shell gland. This can occur in the older hens. Replace single-cycle flocks after 75 weeks of age. | |
| Constant flock agitation or fear response | Choose a site that is isolated to minimize disturbances. Avoid sudden noise. Minimize activities around the hens. Dim the light intensity in the house. Ensure predators do not have access to the hens, hen house, or paddock. | |
| Exposure to chemicals such as BAPN (beta-Aminopropionitrile fumarate), which causes premature passage of the egg through the oviduct | The exposure could come on multi-species farms where BAPN is used to treat older horses for muscle injuries; do not feed to hens or allow access to it. | |
| Reduced calcium intake | Provide increased calcium levels in the diet based on strain, outside temperatures, and feed intake. Supplement diets with oyster shell or crushed calcium as a free-choice option for the hens. | |
| Excessive calcium in the feed (causing sandy shell texture) | Ensure that the diet formulation contains the correct amount of calcium according to the Nutrient Requirements of Poultry, 1994, 9th Edition, National Research Council. | |
| Breed (heredity) | There are strain differences in shell quality. | |
| Porosity (heavily mottled) | Breed of hens | Select a strain bred for good shell texture. |
| Hen age | Sell hens after 12 to 14 months of lay or molt the flock to improve shell structure. | |
| High temperatures, usually associated with the season of the year | Keep the hen house cool; hold eggs in a cool place. | |
| Floating air cell (tremulous or moving air cell) | Rough handling | Handle eggs gently and orient them correctly in the carton. This is not a quality fault when assigning egg grades. |
| Tainted shells with crystalline structures (content off-flavor) | Paradichlorobenzene insect fumigant | Do not feed treated grains to birds. |
| Albumen (Egg white) | ||
| Defect | Cause | Corrective Measure |
| Pink | Cotton seed meal | Avoid using it in the laying hen diet. |
| Yellow coloring in albumen | Hepzide | Do not feed to hens in lay. |
Viscosity
|
Increased alkalinity (pH), loss of CO2 | Quickly refrigerate eggs at or below 45°F. Use an approved shell coating, such as egg oil. |
| Respiratory diseases: Newcastle disease, infectious bronchitis (IBV), laryngotracheitis (LT), and Mycoplasma gallisepticum (MG) | Prevention: Follow the recommended vaccination program during rearing; include periodic booster vaccinations. | |
| Breed (heredity) | Select strains of known albumen production quality. | |
| Arasan (tetramethylthiuram disulfide) | Do not feed or allow hens access to grains treated with Arasan; law requires treated seeds to be dyed. | |
| Vanadium | Use sources of phosphorus in feeds known to have little to no vanadium. | |
| Age of hens | Replace hens after 75 weeks of age. | |
| Ammonia | Improve ventilation; use a litter or manure amendment; remove droppings frequently. | |
| High temperatures | Collect eggs 3 to 5 times per day; hold in refrigerated temperatures (at or below 45°F). | |
| Sulfanilamide (sulfa drugs) | Use according to directions or veterinary prescription. | |
| Noncovered eggs cartons | Utilize enclosed egg cartons to slow gas exchange through pores. | |
| Partially cooked | Avoid excessive heat when washing eggs. | |
| Flecks or spots in albumen after cooking | Protein inclusions colored with pigment caused by porphyrin, which is found in brown-shelled eggs | Select a strain known for producing clear eggs. |
| Early growth of mold in shell | Be sure to collect eggs in a timely manner and refrigerate them quickly. Segregate floor eggs from regular collection. | |
| Cloudy white |
Not a defect—it is associated with very fresh eggs or eggs held in a way that minimized CO2 loss |
Eggs should be stored at or below 45°F as soon as possible after lay. |
| Green rot and other types of microbial spoilage | Bacteria, molds, and fungi from eggs that may have been laid in the litter or on the range paddock and not found on the day of lay Also found in unwashed eggs stored before processing. Poultry fecal matter and poultry house dust are excellent transfer points for bacteria |
Maintain clean nesting materials; gather eggs 3 to 5 times a day. Be sure to collect and dispose of floor eggs. Use clean water for washing eggs; maintain the egg wash water temperature at 90°F, or 20°F warmer than the warmest egg. Use recommended amounts of detergents and sanitizers; keep equipment clean. Check wash water for iron content (it should be below 2 ppm) |
| Example: Green rot (Pseudomonas fluorescens) | Detected with ultraviolet lamp candler; other types of advanced spoilage are easily detected with regular candling techniques. Egg wash water containing 2 ppm of iron or more could promote bacterial growth by deactivating natural antimicrobials in the albumen. | |
| Off-odors and off-flavors | Chemicals for treating parasites | Use chemicals recommended for the control of lice and mites. Do not use materials capable of imparting odors or flavors to eggs, such as Beta-hexachlorocyclohexane (BHC), lindane, or hexaphine. |
| Odorous flowers, fruits, or vegetables in egg storage areas | Do not store strongly scented flowers, fruits, or vegetables in the same area with eggs. Due to the natural respiration of an egg, scents and odors are easily transferred to the egg contents. | |
| Blood and meat spots | Before and during ovulation there may be hemorrhaging (capillary crossing the stigma) | Maintain a tranquil environment. Hens that are scared or stirred up prior to ovulation are more likely to produce increased blood spots. A vitamin pack with vitamins A and K may be given. Aureomycin may be given. |
| Breed (heredity) | Select strains with a low incidence of this problem. Brown egg strains have an approximately 25% greater incidence rate. | |
| Pigmented protein inclusions | Strain related; it’s more prevalent in brown egg strains. | |
| Extended periods of intermittent light | Use 16 hours of light. | |
| Protein inclusions colored with pigment caused by porphyrin, which is found in brown-shelled eggs | Select strains with low incidence of this problem. | |
| Yolk | ||
| Olive or salmon-colored | 5% or more cottonseed meal | Avoid use in laying hen diets. |
| Platinum (colorless) | Possible infection | Consult a veterinarian for an antibiotic to treat the infection. |
| Pale color | Lack of xanthophyll or use of small grains lacking pigment | Increase xanthophyll content in the diet using items such as corn, corn gluten, alfalfa leaf meal, or marigold petals. Feed recommended levels of xanthophyll-bearing materials for desired yolk color. Yellow = 13 mg of xanthophyll per pound of feed. Medium orange = 23 mg of xanthophyll per pound of feed. Orange = 34 mg of xanthophyll per pound of feed. The maximum color will occur 10 days after the hens are placed on feeds for yolk color. |
| Green | 100 to 250 mg of sodium chlorophyllin in feed | Avoid feeding to hens. |
| Seed pods of Capsella bursapastoris (Shepherd’s purse) or Thlaspi arvense (field pennycress) | Use clean grains in feeds; clean weeds from range paddocks. | |
| Greenish-brown | 5 gm or more of pimento peppers fed daily to each hen | Use smaller amounts for a desirable color in egg yolks. |
| Orange-pink | Red pepper | Avoid feeding to hens. |
| Yellow to orange | Hens eating substances such as sea weed (algae); dehydrated alfalfa meal; corn gluten meal; flower petal meal; dried chili peppers; powdered African red peppers; dried sweet potatoes; dried carrots; corn-oil products, and food-grade, fat-soluble dyes | Increase xanthophyll content in the diet using items such as corn, corn gluten, alfalfa leaf meal, or marigold petals. Feed recommended levels of xanthophyll-bearing materials for desired yolk color. Yellow = 13 mg of xanthophyll per pound of feed. Medium orange = 23 mg of xanthophyll per pound of feed. Orange = 34 mg of xanthophyll per pound of feed. The maximum color will occur 10 days after the hens are placed on feeds for yolk color. |
| Misplaced egg yolk |
If small end of the egg is up, with yolk in the large end—egg albumen is thin and/or the yolk has a high fat content |
Practice acceptable gathering, packaging, cooling, and storage of eggs, both pre- and post-processing. Place eggs small end down in the carton. |
| Blood and meat spots | Ovarian hemorrhages—this tendency may be inherited | Select strains with a low incidence of this problem. |
| Mottled or blemished yolks | Nicarbazine | Do not feed to layers. |
| Cottonseed meal | Avoid use in layer diets. | |
| Piperazine citrate | Do not use frequently or continuously. | |
| Movement of water from the albumen across the vitelline membrane into the yolk | Cool eggs quickly and keep them cool. Collect eggs 3 to 5 times per day, and collect floor eggs daily. | |
Viscosity
|
Crude cottonseed oil (malvalic acid and sterculic acid) | Avoid use in layer diets. |
| Yolks laid internally, which result in a distended abdomen and an upright posture over time | Remove these hens from the flock. | |
| Freezing of intact eggs (27°F or below) | Maintain shell egg storage at 45°F or below, but higher than the freezing point. | |
| Off-odors and off-flavors | Insecticides for external parasites Strongly scented fruits and vegetables in the storage area |
Always keep insecticides and other chemicals in a separate area safely away from food items. Do not store eggs with pungent items. |
| Chemicals or washing compounds | Follow the manufacturer’s recommendations for use. | |
| Flat | Weak vitelline membrane Older hens produce eggs with a weaker vitelline membrane |
Gather eggs 3 to 5 times per day and cool them quickly to 45°F. This slows water movement from the albumen into the yolk. |
| Stuck yolk | Newcastle disease | Vaccinate the flock properly. |
| Storage at a high temperature | Gather eggs 3 to 5 times per day and cool them quickly to 45°F. | |
| Extended storage time | Market eggs promptly and practice proper inventory rotation. | |
| Large temperature shifts | Consistently maintain the egg storage temperature at or below 45°F. Avoid large shifts in the egg temperature. | |
| Other Egg Abnormalities | ||
| Egg within an egg | Reverse peristalsis in the oviduct | Reduce hen stress, particularly sudden, acute stressors |
| Soft shell or shell-less (membrane) | Incomplete or not deposition of calcium for shell formation Low dietary calcium |
Calcium should be at or above NRC recommendations of 3.25%, but commercial strains need even higher levels. |
| Diseases such as avian influenza, respiratory infections, Newcastle disease, or egg drop syndrome | Follow the recommended vaccination program during rearing; include periodic booster vaccinations. | |
| Fear or stress can trigger early oviposition of an egg at any point in shell formation | Choose a site that is isolated to minimize disturbances. Avoid sudden noise. Minimize activities around the hens. Dim the light intensity in the house. Ensure predators do not have access to the hens, hen house, or paddock. | |
| Some hens have a genetic disposition to produce shell-less eggs | Select a strain known for good shell quality. | |
Anderson, K. E. 2015. Final Report of the Thirty Ninth North Carolina Layer Performance and Management Test. Vol. 39, No.5. December.
Beyer, R. S. 2005. Factors Affecting Egg Quality. Kansas State University Agricultural Experiment Station and Cooperative Extension Service, EP-127, March.
Davis, J. J. 1921. “Scientific Note: Effect of Feeding Paradichlorobenzene-Treated Feed to Poultry.” J of Economic Entomology 14: 509.
Fitzsimmons R. C., M. Newcombe, and I. E. Moul. 1989. “The Long-Term Effects of Feeding Ground and Whole Cottonseed to Laying Hens.” Can J Anim Sci 69: 425–429.
Gerber, N. 2012. Factors Affecting Egg Quality in the Commercial Laying Hen: A Review. Egg Producers Federation of New Zealand Inc. Poultry Industry Association of New Zealand. 96 D Carlton Gore Road, Newmarket, 1023, Auckland.
King’ori, A. M. 2012. “Egg Quality Defects: Types, Causes, and Occurrence: A Review.” J Anim Prod Adv 2(8): 350–357.
Lorenz, F. W., H. J. Almquist, and G. W. Hendry, 1933. “Malvaceous Plants as a Cause of ‘Pink White’ in Stored Eggs.” Science 77: 606.
Nagalakshmi D., S. V. R. Rao, A. K. Panda, V. R. B. Sastry. 2007. “Cottonseed Meal in Poultry Diets: A Review.” J Poult Sci 44: 119–134.
National Research Council. 1994. Nutrient Requirements of Poultry: 9th Edition.
Phelps, R. A., 1966. “Cottonseed Meal for Poultry: From Research to Practical Application.” World’s Poultry Sci J 22: 86–112.
Ryu, K. N., H. K. No, and W. Prinyawiwatkul. 2011. “Internal Quality and Shelf Life of Eggs Coated With Oils from Different Sources.” J Food Sci 76 (Jun–Jul): S325–9. doi: 10.1111/j.1750-3841.2011.02177.x.
Sherwood, D. H. 1958. “Factors Affecting Egg Quality—A Review.” Poultry Science 37: 924–932.
Tarver, F. R. and T. A. Carter. 1979. Factors Affecting Shell-Egg Quality. AG-169. Raleigh, NC: The North Carolina Agricultural Extension Service. (Revised from Folder 228).
U.S. Department of Agriculture-Agricultural Marketing Service. 2000. Egg-Grading Manual. Agricultural Handbook Number 75. USDA-AMS-Poultry Programs-STOP 0259 1400 Independence Avenue, SW, Washington, DC 20250-0259.
Waldroup P. W. 1981. “Cottonseed Meal in Poultry Diets.” Feedstuffs 53: 21–24.
As part of its nutribiotic approach to feed strategy, Axtra® PHY helps producers improve animal performance, reduce phosphorus waste and overall environmental footprint.
DuPont Animal Nutrition, a business unit of DuPont Nutrition & Biosciences (DuPont), today announced the launch of Axtra® PHY in Japan. DuPont™ Axtra® PHY is the fast-acting phytase enzyme designed to improve animal performance and reduce phosphorus waste.
Axtra® PHY is the fastest route to top animal performance in poultry and swine, while improving profitability for producers and reducing phosphorus waste. The phytase enzyme works quickly in the digestive tract to break down phytate, the main storage form of phosphorus contained in plants and a key anti-nutrient. By lowering the impact of phytate, Axtra® PHY increases the availability of phosphorus, energy and amino acids naturally present in the feed, meaning the producer needs to add fewer costly ingredients. Reducing phosphorus waste also reduces the amount of phosphorus runoff that may pollute nearby surface and groundwater systems. This helps producers operate more sustainably by reducing their overall environmental footprint.
“DuPont offers innovative enzymes that are not only the fastest-acting in market, but also enhance the interaction of nutrition, the microbiome and gut and immune function, improving the nutribiotic state of the gut.”Aart Mateboer, Business Unit Director, DuPont Animal Nutrition
“DuPont offers innovative enzymes that are not only the fastest-acting in market, but also enhance the interaction of nutrition, the microbiome and gut and immune function, improving the nutribiotic state of the gut,” said Aart Mateboer, Business Unit Director, DuPont Animal Nutrition. “We are proud to be the first company to launch a new generation phytase enzyme in the Japanese market.”
Reduction of the anti-nutrient phytate factors leads to improved gut health for swine and poultry. Axtra® PHY also leads to improved weight gain and feed efficiency, helping producers save on feed costs and improving their profitability. DuPont provides optimized dosing that is specific to species, diet and life stage, leading to maximized return for customers.
The launch of DuPont™ Axtra® PHY in Japan follows the 2017 launch of phytase enzyme Phyzyme® XP.
DuPont is investing in science and innovation to help producers improve performance, increase livability and support welfare in the face of increasing pressure to reduce or remove antibiotics from production systems. To learn more, visit animalnutrition.dupont.com/AxtraPHY.
Source: Poultryhealthtoday.com
Successful Salmonella control throughout live poultry production requires an integrated effort, attention to detail and careful follow-through to help processing plants meet more stringent USDA standards, said poultry health experts at a recent food-safety news conference.
“Everyone in live production has to be on board because a slip-up in one area can wreck all the time and effort put into the rest of the plan,” said Don Waldrip, DVM, senior technical services veterinarian, Zoetis.
Charles Hofacre, DVM, PhD, president of the Southern Poultry Research Group Inc., Athens, Georgia, advised starting with clean broiler breeders because hens can vertically pass Salmonella on to their broiler progeny.
“Make sure you know your pullet source and make sure there isn’t a mouse or darkling beetle infestation. I’ve worked with pullet farms where we’ve had to completely tear out sidewalls and insulation to get rid of beetles to get control of Salmonella,” he said.
Vaccination of breeders, broilers or both can help lower the load of Salmonella going into the processing plant and is especially worth considering for companies with plants in Category 3, both veterinarians said. However, they cautioned that Salmonella in flocks might be more than vaccines can handle if not supplemented with other control measures.
Vaccination can be supplemented, Hofacre said, with the use of products like essential oils, probiotics and organic acids.
“They don’t have a direct effect on Salmonella like a vaccine would have, but I think they stimulate intestinal immunity or make the gut a place that’s less favorable for Salmonella colonization,” he explained.
“It usually requires the use of more than one of these to have any success. An essential oil, for example, might not be very helpful when used alone, but if it’s coupled with the right probiotic or botanical, it may help.”
Waldrip reviewed other steps poultry producers can take as part of an integrated food safety plan targeting Salmonella:
Besides a standard operating procedure for Salmonella control, there has to be a way to ensure compliance. “If you’ve got bait boxes out for rodents, someone has to make sure they’ve got bait,” he said. “That’s the kind of little detail that’s essential for a Salmonella control plan to work.”
Waldrip concluded that “If tackling the Salmonella problem sounds like a Herculean and expensive task, it is. But today’s producers can’t afford to skimp on Salmonella control. If FSIS shuts down your plant, it’ll cost a lot more.”
1. Biosecurity procedures in poultry production. World Organisation for Animal Health (OIE). http://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_biosecu_poul_production.pdf Accessed Jan. 24, 2019.
2. Mallinson E. Poultry house ventilation part of Salmonella control. WattAgNet. https://www.wattagnet.com/articles/28724-poultry-house-ventilation-part-of-salmonella-control Accessed Jan. 24, 2019.
3. Poppe C, et al. Salmonella Contamination of Hatching and Table Eggs: A Comparison. Canadian Journal of Veterinary Research 1998; 62: 191-198.
4. Jones FT. A review of practical Salmonella control measures in animal feed. The Journal of Applied Poultry Research, Volume 20, Issue 1, 1 March 2011, Pages 102–113.
5. National Chicken Council Animal Welfare Guidelines and Checklist for Broilers, page 11. https://www.nationalchickencouncil.org/wp-content/uploads/2017/07/NCC-Welfare-Guidelines-Broilers.pdf , Accessed: Jan. 24, 2019.
6. Gast RK. Frequency and Duration of Fecal Shedding of Salmonella Serovars Heidelberg and Typhimurium by Experimentally Infected Laying Hens Housed in Enriched Colony Cages at Different Stocking Densities,” Avian Diseases 61(3), (3 July 2017). https://doi.org/10.1637/11635-032517-RegR Accessed: Jan. 24, 2019.
7. Da Costa, M, et al. Studies on the interaction of necrotic enteritis severity and Salmonella prevalence in broiler chickens. Abstract, annual meeting, American Association of Avian Pathologists, 2018.
8. Watkins, Susan. Effects of Water Acidification on Broiler Performance. The Poultry Site. 29 October 2004. http://www.thepoultrysite.com/articles/246/effects-of-water-acidification-on-broiler-performance/ Accessed January 24, 2019.
9. Ramirez, A, et al. Effect of Feed Withdrawal on the Incidence of Salmonella in the Crops and Ceca of Market Age Broiler Chickens. 1997 Poultry Science 76:654–656.
In-package cold plasma allows to effectively control spoilage organisms in chicken breast.
Cold plasma has minimal effect on colour, pH and water holding capacity of poultry meat.
Plasma treatment allowed to extend the microbial shelf-life by approximately 6 days under refrigerated storage.
In-package cold plasma is a viable alternative to chemical disinfection of poultry surfaces.
Atmospheric cold plasma (ACP) is a promising non-thermal technology for controlling food spoilage. In this study, ACP treatment at 100 kV for 1, 3 and 5 min was applied to chicken breast samples. Approximately 2 log CFU/g reduction in natural microflora of chicken was achieved within 5 min of treatment and 24 h of storage. The observed reduction was attributed to the reactive oxygen and nitrogen species in cold plasma. For shelf-life study, control and ACP treated samples (100 kV for 5 min) were analysed for the population of mesophiles, psychrotrophs and Enterobacteriaceae as well as sample colour and pH over a storage period of 24 days. On day 24, the population of mesophiles, psychrotrophs and Enterobacteriaceae in treated chicken was respectively 1.5, 1.4 and 0.5 log lower than the control. These results suggest that in-package ACP is an effective technology to extend the shelf-life of poultry products.
Fresh poultry products including chicken breast meat are highly perishable. Atmospheric cold plasma is a promising non-thermal technology for controlling food spoilage and extending shelf-life. This work demonstrates the efficacy of in-package cold plasma treatment in decontaminating chicken breast surface and extending the shelf-life. Being an energy efficient and chemical free decontamination intervention, in-package cold plasma is a promising alternative traditional chemical disinfection of poultry surfaces.
The popularity of poultry meat has been steadily increasing around the world over the last few years. Of the various poultry cuts, the consumption of chicken breast fillets is becoming quite popular due to the relatively low cost of production of a high protein food with low fat content and the convenience of a ‘boneless’ meat portion. Due to the perishable nature of chicken meat, the poultry industry remains concerned about shelf-life extension methods for poultry products. The microflora in poultry meat starts off on its surface and their population at any time-point depends on the initial counts and storage conditions. It is well established that this bacterial growth and its activity on the surface of the product are the main causes of the spoilage and unacceptability of chicken products among consumers, resulting in industrial economic losses (Fernández-Pan, Carrión-Granda, & Maté, 2014). In general, whole poultry carcasses are known to carry lower microbial populations than cut-up poultry. An increase in the mean surface counts of microflora from 3.30 log CFU/cm2 to 3.81 log CFU/cm2 after cutting-up, and to 4.08 log CFU/cm2 after packaging was reported long back (May, 1962). To decrease the microbial populations, poultry processors employ a variety of sanitizing treatments, including the use of organic acids (e.g. acetic, lactic, citric, and succinic), chlorine, chlorine dioxide, trisodium phosphate, and acidified sodium chlorite (Keener, Bashor, Curtis, Sheldon, & Kathariou, 2004). However, the increasing consumer concern about the use of chemical based (or chemical ‘sounding’) sanitizers is forcing poultry processors to look for alternative decontamination methods.
Among the emerging innovative antimicrobial technologies, cold plasma is being extensively explored for its surface decontamination capabilities. Cold plasma is essentially an ionised state of a gas achieved by exposing the gas (air or any gas mixture) to very high electric field strengths. The ionised gaseous chemical species include the positive and negative ions, radicals, electrons, excited and neutral molecules, and quanta of electromagnetic radiation (i.e. ultraviolet and visible light) (Fukuda, Kawasaki, & Izawa, 2019). As an example, humid air plasma would contain reactive oxygen and nitrogen species (RONS) such as ozone, singlet oxygen, peroxide, and several types of nitrogen oxides (NxOy). Most of the RONS possess strong antimicrobial activity. These RONS species render cold plasma into an excellent surface decontamination technology.
In an earlier study, Dirks et al. (2012) had shown that plasma application to the surface of raw chicken breast allowed to decrease the natural microflora by 0.85 log units. Similarly, Lee et al. (2016) reported about 1.2 log CFU units reduction in the population of total aerobic bacteria on chicken breast after 5 min of plasma treatment using a flexible thin layer DBD plasma set-up. Recently, Misra and Jo (2017) had reviewed the application potential of cold plasma technology in the meat industry with great attention to the details for effective decontamination. Cold plasma has several advantages with respect to poultry processing, including the ability to combine with other hurdle approaches such as controlled release packaging (Pankaj et al., 2017), essential oil treatments (Chouliara, Karatapanis, Savvaidis, & Kontominas, 2007), refrigeration, and modified atmospheric packaging (Misra et al., 2014). Moreover, cold plasma could overcome the limitations of some approaches such as the requirement for use of large quantities of essential oils to observe reasonable control of the microflora population.
Among the various cold plasma configurations, in-package cold plasma technology is a distinct approach involving the ionization of gas contained in a sealed package in presence of the food product. For in-package plasma treatment, the product is first sealed inside a plastic (or occasionally glass) packaging in air or a modified gas, and the package is exposed to a strong electric field. The ionised gaseous species have a high diffusivity and long lifetime, thus ensuring a uniform treatment of the product. However, being quasi-stable, the reactive species eventually recombine to form the original gas. Thus, the process begins with a known gas mixture (or air) and ends with the same gas mixture after few hours, while effectively eliminating a significant population of the spoilage microorganisms (Misra, Yepez, Xu, & Keener, 2019).
In this work, we have studied the in-package cold treatment of chicken breast in air at a high voltage of 100 kV. The objectives of this study were to (1) evaluate the efficacy of high voltage in-package cold plasma treatment at 100 kV for various treatment times in decreasing the natural microflora in chicken breasts; (2) measure the changes in quality parameters, viz. colour, pH, lipid oxidation, and water holding capacity; (3) study the long-term effects of in-package cold plasma treatment on the native microflora population and quality of chicken breasts stored under refrigerated conditions.
Cylindrical chicken breast samples were obtained via coring-out sections from dorsal side of chicken breasts using a sterile hollow stainless-steel cylinder (height ~ 28 mm, diameter ~ 23 mm) with sharp edges. For all experiments, fresh chicken breast sample (10 g) with no specificity to dorsal or ventral side facing the top was placed at the centre of a polypropylene rigid package with dimensions 168 mm × 120 mm × 28 mm (ArtBin® box). The rigid package was further placed inside a secondary package comprising of a high barrier polypropylene film (Cryovac®). Prior to heat sealing, the package was flushed with dry air (<5% relative humidity) for 2 min. The samples intended for use as control, as well as those for plasma treatment were packaged under the same condition. Samples meant for microbiological analysis were plasma treated separately from those for quality. This method prevented any chances of contamination of samples, that could possibly result from handling during quality testing, that in turn could have affected results of microbiological analysis.
A detailed, labelled schematic of the experimental set-up is provided in Fig. 1. The plasma source employed in for the experiments comprised of a dielectric barrier discharge (DBD) with a step-up transformer providing the power. The step-up transformer operated with an input voltage of 120 V at 60 Hz frequency that could be varied using a voltage regulator. The output from the secondary winding of the step-up transformer was connected to two circular aluminium disc electrodes (outer diameter = 152 mm) separated by 42 mm. The dielectric barriers comprised of 10 mm plexiglass under the powered (high voltage) electrode and a 4 mm polypropylene sheet above the ground electrode. It may be noted that the polypropylene package itself acted as a dielectric. The dielectric layers prevent a transition of the filamentary discharge into an arc, thereby ensuring homogeneity of the plasma treatment. The electrodes were pre-cooled to 5 °C prior to sample treatment using two ice packs. The electrode temperatures were maintained close to 5 °C throughout the plasma treatment process, as measured using an infrared thermometer with an accuracy of ±1 °C (Fisherbrand™ Traceable™, Fisher Scientific, USA), by leaving the icepacks in contact with the electrodes. For the experiments looking at effect of time, the samples were treated at 100 kV for 0, 1, 3, and 5 min durations. The system consumed an average power of 233 ± 5 W for operating at 100 kV, as measured using a power meter (P4460 Kill A Watt, P3 International; procured from Grainger, USA). After plasma treatment, all samples were stored in sealed condition at 4 °C for 24 h prior to microbial recovery and quality measurements.

Fig. 1. Experimental set-up used for plasma treatment of the chicken samples. (colour online).
The arrangement for OES is shown in Fig. 1. The light emitted from the electrical discharge in the package was captured using a 5 mm diameter collimating lens directed towards a solarized optical fiber with a core diameter of 1000 μm. The distance from the collimating lenses to the edge of the box containing sample was set at 7 cm. The optical fiber directed the light to a computer controlled, custom-built Ocean Optics spectrometer (Ocean Optics, Inc., Florida, USA) with 0.2 nm/pixel resolution. The grating spectrometer had a 10 μm slit width with an optical resolution of 0.88 nm. The spectrometer was pre-calibrated by the manufacturer using a mercury‑argon atomic line source. Measurements were carried out over the wavelength window of 200–800 nm of the electromagnetic spectrum. The integration time for spectral recording was set to 2 s and an average of 10 spectrum was recorded, thereby maximizing the signal to noise ratio. Each spectrum was corrected by the software (Ocean View, Ocean Optics, FL, USA) for dark current and the background noise via subtraction, and the averaged spectra were reported. The characteristic molecular and atomic transitions associated with the spectral bands and lines were identified using published reports and National Institute of Standards and Technology (NIST) database (Misra et al., 2014; Misra, Keener, Bourke, Mosnier, & Cullen, 2014).
The objective of the microbiological analysis was to quantify the effect of cold plasma treatment on the background bacterial microflora population in the chicken samples. For bacterial enumeration, the chicken sample was aseptically removed from each package and placed into a sterile stomacher bag. Ninety millilitres of sterile 0.1% peptone solution was added to each bag and the sample was pummelled for 3 min using a Stomacher 400 Laboratory Blender (Seward, Worthington, UK) operating at 230 rpm. The resulting suspension was serially diluted (10-fold) in 0.1% peptone and 0.1-ml aliquots were surface plated on appropriate agar media for each microbial group. For the aerobic plate count samples were surface plated on plate count agar (PCA) and incubated at 35 °C for 48 h for mesophiles and at 7 °C for 7 days for psychrotrophs. The Enterobacteriaceae were enumerated by first pour plating samples in tryptic soy agar supplemented with 0.6% yeast extract (TSAYE), followed by holding the TSAYE plates for 1 h at ambient temperature (22 ± 1 °C). Each solidified TSAYE plate was overlaid with violet red bile (VRB) agar and incubated at 35 °C for 24 h. After the incubation periods, the colonies were manually counted, and the bacterial populations were computed using the respective dilution factors. The bacterial populations were reported as log colony forming units (CFU) per gram.
The colour of the control and plasma treated chicken samples was quantified using a Hunter L⁎-a⁎-b⁎ colorimeter (Colour Quest XE Hunter Lab, Northants, U.K.). The observer angle was set at 10° and the light source was a standard D65 halogen lamp. Prior to evaluation of the samples, the instrument was calibrated using standard white (L⁎ = 93.97, a⁎ = 0.88 and b⁎ = 1.21) and green (L⁎ = 56.23, a⁎ = 21.85, b⁎ = 8.31) tiles. Colour measurement for each sample was done in triplicate (dorsal, ventral, and lateral sides each) and average values were taken for reporting purpose.
A 1.0 g of cold plasma treated, or untreated sample was homogenised at 16,000 rpm with 9.0 ml spectroscopy grade water (Sigma Aldrich, Product No. 270733-4L) for 30 s. The pH of the sample was measured using an Orion Dual Star pH/SE meter. The pH meter was calibrated with the standard buffer solutions (pH 4.00, 7.00, and 10.00) at room temperature prior to measurements.
The WHC of meats is measured via the amount of free water released by the meat after physical pressure or force is exerted upon it. The WHC was determined using a centrifugation method adopted from Zhang and Barbut (2005) and Wardlaw, McCaskill, and Acton (1973). About 5 g of finely chopped chicken breast sample were mixed with 8 ml of cold 0.6 M sodium chloride solution and homogenised for 1 min at 8000 rpm (UltraTurrax T25 homogenizer) followed by incubation at 4 °C for 30 min. After incubation, the meat slurry was again stirred for 1 min followed by centrifugation at 16,000 g for 20 min. The supernatant layer was collected and measured by volume. The amount of added solution retained by the meat is reported as the water holding capacity in ml per 100 g meat.
The method for lipid oxidation measurement by 2-Thiobarbituric Acid Reactive Substances (TBARS) assay was adapted from Botsoglou et al. (1994) and Beltran, Pla, Yuste, and Mor-Mur (2003), with slight modifications. A 2-g chicken breast meat sample was transferred into a 50 ml centrifuge tube with 8 ml 5% aqueous trichloroacetic acid (TCA) and 5 ml 0.8% butylated hydroxytoluene (BHT) in hexane. The mixture was then homogenised at 16,000 rpm for 30 s. The homogenate was centrifuged for 7 min at 15,000 g, and the upper hexane layer was discarded. The 2.5 ml aliquot from the lower aqueous layer was mixed with 1.5 ml of 0.8% aqueous thiobarbituric acid (TBA) followed by 25 min incubation at 80 °C. The tubes were then cooled in running tap water for 10 min and then stabilised at room temperature for 30 min. Subsequently, the absorbance was read at 521 nm using a spectrophotometer (Synergy HT, BioTek Instruments, Winooski, VT). The TBA content was expressed as mg of MDA per kg of chicken using a calibration curve prepared for MDA concentration versus absorbance (see Supplementary information, SI. 1), which was interpolated via linear regression.
Based on the results of the effects of treatment time, a separate storage study was carried out for chicken breast samples treated at 100 kV for 5 min. For the storage studies, both control and plasma treated samples were stored aerobically under refrigerated conditions (4 °C) until further analysis. The stored samples were sampled on days 0 (i.e. after 24 h of in-pack storage), 3, 6, 9, 12, 15, 18, 21 and 24 for microbial enumeration (mesophiles, psychrotrophs, and Enterobacteriaceae), and quality studies. Due to time constraints and the labour involved, only colour and pH were the quality characteristics measured for the storage study.
The evolutions of microbial populations over the storage period was fitted to an empirical logistic bacterial growth model and the growth rate parameter obtained was used for comparing the growth of microbial populations in control versus plasma treated chicken samples. The logistic growth model is described by Eq. (1) (Pankaj, Misra, & Cullen, 2013).(1)
where N(t) is the population at any time t, N0 (log CFU/g) is the initial population, Nasymp (log CFU/g) is the population number approached at the stationary phase (which is an asymptote), k (h−1) is the rate constant, and tc (h) is a marker of the inflection point. One could in practice, employ any of the several models available for microbial growth modelling (Peleg & Corradini, 2011), and not necessarily the logistic type of growth model. We chose the logistic model for the relative simplicity of this model and ease of parameter interpretation. The fitted models were used for predicting the time to reach the cut-off values for each microbial group.
The experiments evaluating the effects of treatment time were repeated thrice and each set was replicated five times, thereby resulting in 15 measurements. For the storage studies, all experiments were performed in triplicates. Statistical analysis and plotting of data were carried out through scripts written in the open-source R programming software (R foundation for statistical computing, Vienna, Austria). Analysis of variance (ANOVA) was used for mean comparison and the data was represented as mean value ± standard deviation. Statistical significance was evaluated at p ≤ 0.05. The fitting of experimental microflora evolution data during storage to the logistic model was done through nonlinear regression using the Levenberg–Marquardt algorithm for least—squares optimization, and the goodness of fit was evaluated via the coefficient of regression (Radj2) and the Root Mean Square Error (RMSE) statistics.
The chemistry of plasma discharges in air has been widely recognised to be very complex, with the involvement of over 75 species, that interact via over 1000 reaction schemes (Misra et al., 2018). Furthermore, these reactions are multiscale in nature, occurring over a range of length and time scales. Emission spectroscopy of plasma allows study of the nature of the excited species in plasma. The emission spectra for the air plasma in presence of the chicken samples is shown in Fig. 2. The spectrum reveals the presence of strong emissions in the wavelength range of 315–405 nm corresponding to electronic transitions from nitrogen second positive system, N2(C-B) and first negative system, N2+ (B-X). The band heads of the N2(C3Πu → B3Πg) second positive system were recorded around 336.9 nm, 357.3 nm, 380.0 nm and 405.4 nm, while the spectral emission of the nitrogen mono-positive ion N2(B2∑u+ → X2∑g+) was recorded at 390.6 nm and 427 nm with relatively low intensities. The intense spectral signatures indicated the occurrence of energetic collisions of electrons with molecular nitrogen in air. During the electrical discharge process, electrons acquire enough energy (temperature) to ionize dominant air molecules (N2 and O2) and form the excited N2* molecules:

Fig. 2. Optical emission spectrum of the plasma discharge inside the sealed package in presence of chicken breast. (colour online).
Fast quenching of the excited N2* molecules with molecular oxygen is one of the sources of atomic oxygen:
However, the relatively lower intensities of OES peaks associated with oxygen is due to self-quenching of O(3P) in the air plasma (Walsh, Liu, Iza, Rong, & Kong, 2010):
The singlet oxygen formed in plasma also undergoes self-recombination or react with ozone (O3) to produce molecular oxygen:
Ozone is a well-known antibacterial species that forms in the plasma discharges, and has also been measured earlier using chemical analysis and optical absorption spectroscopy at significant concentrations with the DBD system employed in our experiments (Mahnot, Mahanta, Keener, & Misra, 2019; Moiseev et al., 2014).
Microorganisms are often classified according to their growth temperature as either thermophiles (growth temperature: >50 °C), mesophiles (growth temperature: from 20 °C to 50 °C), or psychrophiles (growth temperature: <20 °C). With respect to the current study, evaluations of the numbers of mesophiles and psychrotrophs are important with regard to the spoilage of chicken breast meat intended for human consumption. The Enterobacteriaceae are a group of Gram-negative bacteria whose population is often considered a hygiene indicator during poultry processing (Chouliara et al., 2007). With respect to mesophiles, psychrotrophs, and Enterobacteriaceae, the mean counts were 4.5 log cfu/g, 4.6 log CFU/g, and 3 log CFU/g, respectively, for untreated samples (0 min and 24 h storage), indicative of good quality chicken meat (see Fig. 3). After 5 min of treatment at 100 kV and 24 h in-package storage, the mean populations of mesophiles, psychrotrophs, and Enterobacteriaceae were found to have decreased to 2.6 log cfu/g, 3 log CFU/g, and 1.3 log CFU/g, respectively. Thus, 5 min of plasma treatment allowed to decrease the native microflora by approximately 1.5 log CFU/g. This decrease is higher than the 0.85 log units reported by Dirks et al. (2012), possibly due to rapid production of large concentrations of RONS. However, it is comparable to the 1.5 log units decrease reported by Kronn et al. (2015), who employed a modified atmosphere gas blend (65% O2, 30% CO2, 5% N2) for in-package treatment with a similar DBD plasma system. The treatment time was found to have a significant effect (p < 0.05) on the decrease in population for all three microbial classes. This rapid decrease in population was due to the generation of large concentrations of RONS species inside the package with longer treatment times, which act on the microbial cells resulting in their oxidation and leakage (Misra & Jo, 2017). The increased levels of microbial inactivation with increasing treatment time could also be in-part due to increased susceptibility to inactivation by plasma RONS as a result of the drop in pH (discussed later in section 3.4). It is worthwhile mentioning that our results contradict the reports of Zhuang et al. (2019), who observed that the antimicrobial effectiveness against food-borne pathogens is not influenced by cold plasma treatment time (from 60 s to 300 s) at 70 kV. This is most likely due to the drastically different plasma chemical dynamics at 70 kV versus 100 kV.

Fig. 3. Evolution of the population of (A) aerobic mesophiles, (B) psychrotrophs, and (C) Enterobacteriaceae in chicken breast samples (n = 15) subjected to in-package cold plasma treatments at 100 kV, 60 Hz frequency in air. Populations are presented as log transformations to base 10. The numeric values above each ‘jittered’ time group of data represent the p-value for pairwise mean comparison. The p-value for overall significance of the differences obtained via analysis of variance (ANOVA) is also included. The relative population values are colour coded, and lines of best fit obtained from linear regression are included for ease of visualization only. (colour online).
The change in colour parameters of the chicken breast samples as a function of treatment is summarised in Fig. 4. The L* (Lightness), a* (redness), b* (yellowness) values of plasma-treated chicken breasts were found to be closely related to the untreated control samples, with no statistically significant difference between them (p > 0.05). This insignificant difference persisted treatments up to 5 min followed by in-pack storage for 24 h. The results were consistent for all the three colour parameters (L*, a*, and b*). Our results are in agreement with those reported by Wang, Zhuang, Lawrence, and Zhang (2018), who reported insignificant changes in the all the colour parameters of chicken fillets subjected to cold plasma treatments using a dielectric barrier discharge source operating at 80 kV. However, in an earlier study, plasma treatment using a surface barrier discharge was found to result in greenness and enhanced lightness in chicken breast samples (Lee et al., 2016). A change in colour of other types of meat samples exposed to plasma discharges from different plasma sources (microwave and surface barrier discharge) has been reported in some studies (Fröhling et al., 2012; Jayasena et al., 2015). Such differences are likely related to the plasma chemistry associated with the type of discharge systems used and their operating parameters. For example, Jayasena et al. (2015) employed a surface barrier discharge that is known to have a very different plasma chemistry as compared to a volumetric discharge used in our experiments (Moiseev et al., 2014; Park, Choe, & Jo, 2018). Overall, it becomes clear that the plasma source employed in our study did not lead to any significant change in the colour of the chicken breast samples after treatment.

Fig. 4. Evolution of the colour parameters, viz. (A) lightness, L*, (B) redness-greenness, a*, and (C) blueness-yellowness, b* of chicken breast samples (n = 15) subjected to in-package cold plasma treatments at 100 kV, 60 Hz frequency in air. The numeric values above each ‘jittered’ time group of data represent the p-value for pairwise mean comparison. The p-value for overall significance of the differences obtained via analysis of variance (ANOVA) is also included. The relative values are colour coded, and lines of best fit obtained from linear regression are included for ease of visualization only. (colour online).
We further carried out treatments for extended times of 15 min (while the experiments were only done for 5 min), to record the maximum increase in temperature. For 15 min of operation, the plasma source employed in our study does not lead to an increase in temperature by >7 °C – 8 °C. That said, even a 5 °C to 6 °C rise in temperature of the samples could cause some discoloration of the chicken, especially an increase in the lightness. This was confirmed during our preliminary trials prior to design of the final experimental plan for this work. Recently, Zhuang et al. (2019) have also observed this effect when using a similar plasma source for treatment of chicken breast samples, with no reported cooling of the electrodes. Therefore, preventing a rise in sample temperature throughout the plasma process, via cooling of the electrodes is important for maintaining the quality of chicken breasts. It is worthwhile mentioning that such cooling of electrodes may not very critical when treating other kind of products such as fruits or vegetables (Misra et al., 2014).
The pH of the control and plasma treated samples are shown in Fig. 5a. The pH of control chicken breast samples was found to vary between 5.6 and 6.1 units. An overall decrease in pH of the chicken samples was observed for all treatment times. However, the pH drop was statistically significant (p < 0.05) between 1 min and 3 min of treatment, but not between 3 min and 5 min of treatment. This is likely linked to the plasma chemistry, where much of the NxOy form during the initial 1 min to 3 min of treatment, following which they are dominated by production of or conversion to other reactive species. A decrease in pH of chicken and other meats following exposure to plasma discharge has been reported in several other studies (Kim, Yong, Park, Choe, & Jo, 2013; Rothrock Jr. et al., 2017). Additionally, a significant drop in the pH of aqueous samples exposed to similar type of DBD plasma had revealed a considerable drop in pH (Misra, Keener, Bourke, & Cullen, 2015). In our earlier work, post-discharge, we had confirmed the presence of nitrous species (nitrate and nitrite) in the gas phase (Mahnot, Mahanta, Keener, & Misra, 2019). The decrease in pH is commonly attributed to the formation of very low concentration of nitric and nitrous acid from the dissolution of NxOy species formed in the gaseous phase into the water covering the muscles-.

Fig. 5. Evolution of the chemical quality parameters, viz. (A) pH, (B) Water Holding Capacity, WHC (mL/100 g), and (C) malonaldehyde content (mg/kg), of chicken breast samples (n = 15) subjected to in-package cold plasma treatments at 100 kV, 60 Hz frequency in air. The numeric values above each ‘jittered’ group of data represent the p-value for pairwise mean comparison. The p-value for overall significance of the differences obtained via analysis of variance (ANOVA) is also included. The relative values are colour coded, and lines of best fit obtained from linear regression are included for ease of visualization only. (colour online).
In some studies, however, it has been reported that no change in the pH was recorded in meat samples even after plasma treatment; e.g. for beef loin (Bauer et al., 2017) and bacon (Kim et al., 2011). Such differences are likely linked to the plasma chemistry, the surface moisture content, the humidity in gas, and the buffering ability of the muscle type.
The 70–75% of water in the muscles of live poultry is bound primarily to the muscle proteins. This ability of muscle proteins to intracellularly bind about 90% of the water is referred to as WHC (Honikel, 1987). The WHC of the control chicken breast was found to be close to 24 ml/100 g (see Fig. 5b). This value is in agreement with that reported by Zhuang and Savage (2012). Plasma treatments up to 3 min were found to result in insignificant changes (p > 0.05) in the WHC of the muscle cells. However, the treatment for 5 min was found to result in a significant (p < 0.05) loss of WHC reaching about 17 ml/100 g. It is well-known that a accelerated drop in pH of lean muscles results in a decrease in their WHC, due to a decrease in the space in the myofibril compartment (Huff-Lonergan & Lonergan, 2005). Further, a denaturation of proteins on surface of the chicken samples cannot be overruled. Such denaturation will also result in a decrease in the WHC of the muscles.
Upon oxidation, lipids result in the formation of peroxides, which further decompose to secondary oxidation products, including malonaldehyde (MDA). Therefore, MDA is used as an indicator of lipid oxidation and deterioration in meat and meat products. The TBARS assay involves the condensation of one molecule of malonaldehyde with two molecules of 2-thiobarbituric acid under heated acidic conditions to form a pink chromogen, which was spectrophotometrically measured in the assay. The results of TBARS assay indicated that the difference in mean MDA content was insignificant (p > 0.05) between control and plasma treated samples at all treatment times (Fig. 5c). Our results are in agreement with the findings of Lee et al. (2016) for surface barrier discharge plasma treated chicken breast. In a recent review of the effects of cold plasma on lipid oxidation in meat and meat products, it was reported that chicken breast was more stable to plasma-led oxidation than red meats (Gavahian, Chu, Mousavi Khaneghah, Barba, & Misra, 2018). Such differences can be attributed to the very low-fat content of lean chicken breast as compared to red meat (pork or beef).
The evolution of microbial populations during the storage period are summarised in Fig. 7, and the summary of model fitting is provided in Table 1. The model fitting was found to be adequate with high R2 (adj) values and low RMSE values. Following plasma treatment, the population of all the three microbial groups were significantly decreased by 1 to 1.5 log CFU/g. This was also evident from the modelling parameter N0 (Table 1). All the three groups of micro-organisms, viz. mesophiles, Enterobacteriaceae, and psychrotrophs grew at a slower rate in plasma treated chicken than control, as can be observed from Fig. 6a, b and c), respectively. The model fitting also confirmed that the growth rate parameter k (h−1) was lower for plasma treated microbial group as compared to control for all three groups (see Table 1). Towards the end of storage study, the microbial populations for control and plasma treated samples converged towards nearly the same population levels of ~10 log CFU/g, with the plasma treated samples still exhibiting lower values (see Nasymp parameter in Table 1). The lag phase was found to be significantly extended for mesophiles, and psychrophiles after plasma treatment by >100 h (tc parameter). However, the Enterobacteriaceae were found to have similar lag phase values for control as well as plasma treated samples, thus indicating a quicker recovery from plasma-led injury. However, despite the similar lag phase periods, the population of Enterobacteriaceae remained lower in plasma treated samples vis-à-vis control, throughout the duration of storage study (Fig. 6b).
Table 1. Summary of model parameters for different microbial groups and the statistical parameters of non-linear regression.
| Sample | N0 (log CFU/g) | Nasymp (log CFU/g) | tc (h) | k (h−1) | R2 (adj) | RMSE | |
|---|---|---|---|---|---|---|---|
| Mesophiles | Control | 3.5 ± 0.4 | 10.5 ± 0.1 | 137.6 ± 11.3 | 0.016 ± 0.002 | 0.98 | 0.32 |
| Plasma | 2.0 ± 0.3 | 9.7 ± 0.2 | 243.7 ± 10.5 | 0.010 ± 0.001 | 0.98 | 0.31 | |
| Enterobacteriaceae | Control | 2.8 ± 0.1 | 10.6 ± 0.1 | 246.7 ± 3.2 | 0.040 ± 0.003 | 0.99 | 0.32 |
| Plasma | 1.3 ± 0.2 | 8.9 ± 0.1 | 254.0 ± 5.9 | 0.017 ± 0.001 | 0.98 | 0.34 | |
| Psychrotrophs | Control | 4.1 ± 0.2 | 10.0 ± 0.1 | 137.2 ± 5.3 | 0.030 ± 0.004 | 0.98 | 0.31 |
| Plasma | 2.6 ± 0.2 | 9.6 ± 0.1 | 249.5 ± 7.4 | 0.014 ± 0.001 | 0.98 | 0.32 |

Fig. 6. Evolution of the microbial populations in control (■) and plasma treated (●) chicken stored at 4 °C: (A) Mesophiles, (B) Enterobacteriaceae, (C) Psychrotrophs. The symbols correspond to experimental data, whereas solid lines are curves obtained from fitting of the logistic model via non-linear regression. Populations are log transformations to base 10. (colour online).
When used together, refrigeration and modified atmosphere packaging has been shown to ensure a minimum shelf-life period of 8 days (Chouliara et al., 2007). The recommended cut-off population limits of Enterobacteriaceae and aerobic mesophiles for acceptability of chicken breasts is 7 log CFU/g and 6 log CFU/g, respectively (Chouliara et al., 2007; Fernández-Pan et al., 2014). While no cut-off limits are reported in literature for psychrotrophs, we chose a value of 7 log on a conservative side. In this study, the Enterobacteriaceae population was found to reach 7 log CFU/g on day 10, the psychrotroph population on day 5 and the mesophiles population was found to reach 6 log CFU/g on day 4 for the control samples. For the plasma treated samples, the Enterobacteriaceae and psychrotroph population were found to reach 7 log CFU/g on day 13 and day 12, respectively. The mesophiles population was found to reach 6 log CFU/g on day 10 for plasma treated samples. Thus, by using a simple principle of minimum extension time, cold plasma treatments in air effectively enabled extending the microbial shelf-life by approximately 6 additional days at 4 °C storage temperature. That said, a complete shelf-life evaluation of plasma treated chicken for dynamic conditions should also be confirmed through evaluation of bacterial growth at different temperatures (Mahnot, Mahanta, Farkas, Keener, & Misra, 2019)).
The variations in colour parameters of the chicken breast samples over the storage period are summarised in supplementary file (supplementary information SI 2). The difference in the mean values of green-red (a*) and blue-yellow (b*) parameters were found to be non-significant (p > 0.05) between the control and plasma treated group over the 24 days storage period. However, the b* value was observed to be significantly different (p < 0.05) only on day 15. This is likely due to a natural variability in the colour of those specific samples, as the b* values were found to be insignificant for the subsequent days. Unlike the a* and b* values, the lightness parameter was found to be significantly (p < 0.05) different for control and plasma treated samples after the 9th day of storage. On the 24th day, the difference was again found to be insignificant (p > 0.05). The decrease in lightness (L*) of the control could be attributed to the formation of slime on the surface and spoilage caused by the activity of higher microbial population as compared to plasma treated samples.
The changes in pH of the control and plasma treated samples during storage are summarised in Fig. 7. The difference in pH of the control and plasma treated chicken samples was found to be statistically insignificant until 9th day (p > 0.05). From the 12th day onwards the pH of control was found to increase, which was significant (p < 0.05) as compared to plasma treated samples. The increase in pH of control samples can be correlated with a rapid increase in the population of micro-organisms, and their proteolytic activity results in the formation of basic compounds (Vinci & Antonelli, 2002).

Fig. 7. Change in pH of control and plasma treated chicken breast samples stored at 4 °C. The significance of the difference between mean values is indicated above each day (*’s indicate that the difference was significant, while ns refers to non-significant difference between the mean values). (colour online).
Our work reveals that in-package cold plasma treatment at 100 kV can considerably inhibit growth of spoilage micro-organisms on lean poultry meat surfaces without drastically altering the quality parameters. At least 1.5 log CFU/g reduction in the population of mesophiles, Enterobacteriaceae, and psychrotrophs was confirmed after 5 min of plasma treatment at 100 kV and in-pack storage for 24 h. The variations in the quality parameters between control and plasma treated chicken breast was practically negligible. A storage study under refrigeration revealed that plasma treatment allows to extend the shelf-life of the chicken breasts by 6 days. These effects render in-package cold plasma treatment as a promising technology for extending the shelf-life of poultry meat.
As a next step, exploration of the use of modified gases is encouraged to further decrease the microflora population and increase the shelf- life of poultry meat. In addition, a detailed study of the volatile profile of chicken meat subjected to cold plasma treatment should also be carried out to assess the effects of reactive species in cold plasma.
None.
Authors acknowledge Dr. Buddhi Lamsal, Iowa State University for providing access to the temperature-controlled centrifuge.
Supplementary material.
Huff-Lonergan and Lonergan, 2005
Performance Trace Minerals: Key Component to Antibiotic-Free Poultry Production, Written by Dr. Christof Rapp Zinpro Corporation
There is a growing consumer market looking for antibiotic-free poultry. Total sales of organic food reached $47 billion in 2016, up more than 8 percent from 2015. Sales of organic meat and poultry grew more than 17 percent to $991 million in 2016, marking the category’s biggest yearly gain. The organic sector is still just five percent of overall sales, but it’s growing fast, which presents both pressure and opportunity for poultry producers.
To capitalize on this growing antibiotic-free market trend and produce birds that have a strong immune response to potential pathogen and toxin challenges, poultry producers are turning to performance trace minerals — zinc, manganese and copper — to fortify broilers, broiler breeders and layer natural defenses.
Trace Minerals Build Intestinal and Skin Barriers in Poultry
Trace minerals strengthen animals’ first lines of defense against disease. In the intestine, a single layer of epithelial cells forms the main barrier against pathogens … these tight junctions seal the gaps between the neighboring cells to prevent pathogen or toxin intrusions. Heat stress, dysbacteriosis or a zinc deficiency can loosen the tight junctions, allowing pathogens and toxins to permeate this barrier (Leaky Gut) and reach the bloodstream causing inflammation (Finamore et al., 2009).
The skin also provides a primary defense against disease for all humans and animals. Zinc is a key trace mineral that helps strengthen the integrity of the skin helping it to resist scratches and heal faster in the presence of cellulitis.
In a research study, the percentage of broilers affected by cellulitis was decreased from 52 to 40 percent when the broiler diet was supplemented with 40 ppm of zinc as a zinc amino acid complex. Another study showed improved footpad integrity, with 50 versus 30 percent of birds having normal footpads (Downs et al., 2000; Saenmahayak et al., 2010).
Immune Cell Function in Poultry
If pathogens and toxins manage to cross the epithelial cell barrier and access the bloodstream, then the immune cells, such as white blood cells — a process called immune activation or inflammation — are called into action.
Macrophages, a type of white blood cell, engulf and destroy foreign matter such as bacteria (e.g., Escherichia coli) and cellular debris and, thus, play a crucial role in inflammation. Complexed trace minerals are essential for mounting a proper immune response through the multiple roles they play.
Zinc is essential for the proliferation and proper function of immune cells (Rink and Gabriel, 2000). E. coli cleared faster from the blood of three-week-old turkeys when the diet was supplemented with 40 ppm of complexed zinc (Kidd et al., 1994). Zinc, manganese and copper also help shield healthy cells from becoming damaged as macrophages destroy pathogens.
Trace Mineral Protection for Chicks
Trace minerals also help fortify egg shells, the primary barrier against disease for gestating chicks. Feeding broiler breeders amino acid complexes with zinc, manganese and copper, versus inorganic minerals, has resulted in thicker eggshells (Favero et al., 2013). Also, Hudson et al. (2004) found a decreased number of cracked eggs in broiler breeders fed a zinc amino acid complex compared to eggs from breeders fed zinc sulfate. Feeding zinc and manganese amino acid complexes has also been shown to increase the effectiveness of vaccinations in young birds in antibiotic-free production.
Trace minerals can also positively impact fertility. Breeders fed a zinc amino acid complex containing 80 ppm of zinc (Hudson et al., 2004) or amino acid complexes of zinc, manganese and copper containing 40 ppm of zinc, 40 ppm of manganese and 7 ppm of copper (Favero et al., 2013) produced 2.1 or 2.8 more chicks per hen housed versus breeders fed equal amounts of trace minerals from sulfate sources.
Virden et al. (2002) also improved chick livability by 1.7 percent (100 versus 98.3 percent) in progeny from breeders fed zinc and manganese amino acid complexes versus those receiving equal amounts of inorganic trace minerals — 75 ppm of zinc and 80 ppm of manganese — on top of a diet that was already well fortified with zinc and manganese. In ovo injection of complexed zinc methionine during incubation increased the intestinal surface area of the chick at hatch by 47 percent (Tako et al., 2005), showing the importance of zinc for embryo and early chick development. The larger intestinal surface may partly explain the increased survival found by Virden et al. (2002).
Immunity Booster for Poultry
Antibiotic-free poultry production requires a robust immune system and strong epithelial layer of cells in the gastrointestinal tract and skin lining to help provide a first line of defense against pathogens and toxins that can have a detrimental impact on poultry performance. Zinpro Performance Minerals®, such as zinc, manganese and copper, are essential components to a successful antibiotic-free production system.
For more information on how investing in effective trace mineral supplements can reduce costs and open up new markets for poultry, contact your Zinpro representative.