
The permeability of the intestinal tract controls the uptake of nutrients and the transport of unwanted extracellular substances such as bacteria and xenobiotics, in addition to the nondigested substances. Therefore, gut health plays an essential role in the pathogenesis of various intestinal disorders.
The permeability of the intestine is controlled by gut microbiota, digestive secretions, physical barriers (mucin, intestinal epithelial cells lining and tight junctions), and chemicals such as cytokines.
ALTERATION IN THE INTESTINAL MICROBIOTA
Under normal conditions, the symbiotic relationship between the gut microbiota and the host crucially determines intestinal health. However, a disturbance in the gut microbiota can lead to an imbalanced host–microbe relationship, which is called “dysbiosis”.
- Several factors, such as antinutritional factors in feed, heavy metals, toxic substances, bacterial toxins, herbicides, and antibiotics, can disrupt the gut microbiota.
- These impacts can lead to localized inflammation, extensive infection, or even intoxication.
- Additionally, the intestinal epithelium forms tight connections, acting as a biological barrier that controls the paracellular transit of different materials across the intestinal epithelium, including ions, solutes, and water.
- It also functions as a barrier of extracellular bacteria, antigens, and xenobiotics.
IMPAIRED INTESTINAL BARRIER
The impaired intestinal barrier function, commonly known as “leaky gut”, is a condition in which the small intestine lining becomes damaged, leading to infiltration of luminal contents such as bacteria and their associated components including toxins to pass between epithelial cells.
- These conditions subsequently lead to cell damage and/or inflammation of the intestine, characterized by increased levels of bacteria-derived endotoxins in blood.
- This inflammatory process consumes significant amounts of nutrients, and, subsequently, has negative effects on metabolic responses, in particular on immunometabolic and endocrine responses.
- As a result, animal performances are severely reduced.
Additionally, field observations in Europe showed that the poultry industry faced several problems after the ban of antibiotic growth promoters (AGPs), including negative impacts on performance, animal welfare aspects, and general health issues.
In this review, we discuss the role of these alternatives in maintaining gut function through modulation of the gut microbiota and the related effects benefitting health and quality of poultry.
INTESTINAL MICROBIOTA IN POULTRY
Microorganisms that live in animals’ gastrointestinal tracts (GITs) are a prime example of beneficial bacteria. Indeed, the GIT is the home of a diverse and plentiful microbial community providing essential functions to their host animals.
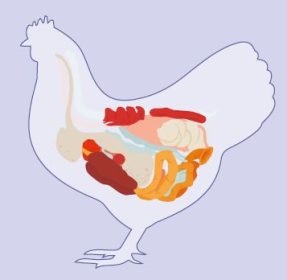
Impact on development and function
Although the intestine is exposed to microflora components from birth or hatching, little is known about their impact on healthy development and function.
- Microorganisms are more densely populated in the GIT than in any other organ.
- Animals have evolved the ability to host complex and dynamic consortia of microbes over their life cycle during millions of years of evolution.
- As a result, a detailed understanding of the contributions of these indigenous microbial communities to host development and adult physiology is required for a thorough comprehension of vertebrate biology.
- Animal species, breed, age, nutrition, environment, rearing forms, stocking density, stress, and medicine can all have an impact on the delicate composition of the gut microbiota. Factors affecting the composition in gut microbiota are shown in Figure 1.
Most of these intestinal microflora’s species cannot be cultured when they are removed from their niches, as is the case with most complex ecosystems.
Colonization of avian guts could already start during embryogenesis and progresses to the formation of a complex and dynamic microbial society.
- Based on principles established during animal history, extensive and combinatorial microbial–microbial and host–microbial interactions are likely to govern the microbiota assembly.
Comparing germ-free rodents that were raised without exposure to microorganisms to those that built up a microbiota since birth, or those that were colonized with microbiota components during or after postnatal development, a variety of host functions influenced by indigenous
microbial communities were identified.
The microbiota, for example:
- Directs the formation of gut-associated lymphoid tissue.
- Aids immune system education.
- Affects the integrity of the intestinal mucosal barrier.
- Modulates proliferation and differentiation of epithelial lineages.
- Regulates angiogenesis.
- Modifies enteric nervous system activity.
- Plays a critical role in extracting and processing the nutrients consumed.
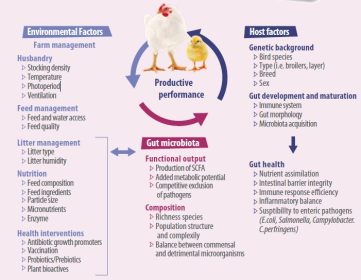
Besides, Proteins and protein breakdown products, sulfur-containing substances, and endogenous or foreign glycoproteins can all be metabolized by the microflora.
- Some bacteria even feed on bacterial fermentation products or intermediates including H2, lactate, succinate, formate, and ethanol and convert them to end products which are again secreted to the gut lumen, such as short-chain fatty acids (SCFA), a process that has a direct impact on gut physiology.
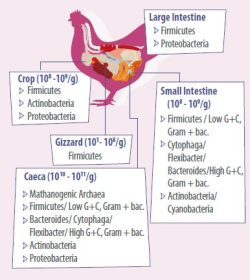
More than 90% of all gut microbiota species in humans and animals belong to the phyla Bacteroidetes, Firmicutes and Actinobacteria, others are Fusobacteria, Proteobacteria, Verrucomicrobia, and Cyanobacteria.
In chickens, the phyla Bacteroidetes and Firmicutes are the most predominant representatives in the gut. In human and several animals, the ratio between Firmicutes and Bacteroidetes is a health/metabolism-associated marker.
- Firmicutes species decompose polysaccharides and produce butyrate.
- Bacteroidetes species degrade complex carbohydrates and synthesize mainly propionate.
- The mechanisms by which bacteria exert effects on the gastrointestinal tract are largely unknown, but manipulation of these triggers is considered to be a promising mean to achieve optimal health and performance.
Nutraceuticals
It is also assumed that the molecular principles that aid in the modification and maintenance of normal physiological functioning of the gut microbiota are mainly derived from food and its supplements, such as nutraceuticals.
Nutraceuticals can include everything:
- Isolated nutrients (vitamins, minerals, amino acids, fatty acids);
- Herbal goods (polyphenols, herbs, spices);
- Dietary supplements (probiotics, prebiotics, synbiotics, organic acids, antioxidants, enzymes); and
- Genetically modified foods.
These nutraceuticals also aid in the prevention of infectious diseases of the host.
Additionally, several multidrug resistance bacteria have emerged making this crisis global. Nutraceuticals will be required to reduce the use of antibiotics.

Lactic acid bacteria have been used as feed supplements since pre-Christian times when humans ingested fermented milk.
- This subject was not analyzed scientifically until the last century, when Eli Metchnikoff (1845–1916), working at the Pasteur Institute in Paris, discovered a link between human longevity and the importance of maintaining a healthy mix of beneficial and pathogenic microbes in the gut.
- Metchnikoff and his co-workers identified the ‘Bulgarian bacillus’, most likely Lactobacillus bulgaricus, which was employed in later trials.
- Today, this microorganism is known as Lactobacillus delbrueckii subsp. bulgaricus, which is one of the bacteria that is used to ferment milk and make yogurt. Following Metchnikoff’s death in 1916, the focus of work in this field shifted to the United States.
- In the late 1940s it was discovered that antibiotics added to farm animals’ feed aided their growth. The need to understand the mechanisms behind this impact prompted more research into the composition of the gut microflora and how it can affect the host animal health.
Progress in bacteriology and the easier availability of germ-free animals helped to assess the impact of newly identified intestinal occupants on the host.
- Based on these studies, it became clear that Lactobacillus acidophilus was not the only Lactobacillus in the intestine, and a variety of other species were examined and eventually included in probiotic formulations.
- The main representatives in gut microbiota of chickens are summarized in Figure 2. Understanding how the intestine matures and develops in chickens and how feed supplements benefit the gut performance will increase feed efficiency, growth, and overall health.
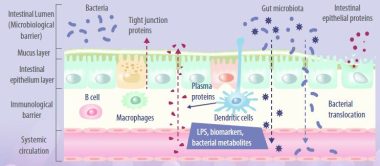
INTESTINAL BARRIER AND TIGHT JUNCTIONS
Enterocytes are the cornerstone of the intestinal mucosal monolayer that protects the host from the external environment. A scheme of the intestinal epithelial barrier and some interactions with intestinal microbiota is shown in Figure 3.
- Enterocytes are connected by the so-called tight junctions (TJs), which constitute a continuous belt of intimate contacts formed during the assembly process of integral transmembranes (occludin, claudins, junctional adhesion molecules (JAMs), and tricellulin) and peripheral membranes (zonula occludens-1 (ZO-1), ZO-2, and ZO-3).
- The TJ proteins are located between adjacent enterocytes, sealing the paracellular space and regulating the permeability of the intestinal barrier.
- Therefore, these proteins prevent the transit of microorganisms, toxins and other antigens from the intestinal lumen to the systemic circulation.
The formation and function of tight junctions are controlled by intracellular signal transduction pathways:
- Protein kinase C (PKC), A (PKA), and G (PKG) signaling,
- Phosphatase-Rho, myosin light chain (MLC) kinase (MLCK), MAPK signaling, and
- The PI3K/Akt pathway.
The disruption of tight junctions by bacterial factors can occur in the following steps:
- Bacterial lipopolysaccharide (LPS) activates the intestinal epithelial cells and macrophages;
- These cells secrete proinflammatory cytokines such as IL-1ß; and
- IL-1ß further activates these cells and triggers intracellular signaling, such as p38 MAP kinase, which subsequently activates MLCK. Finally, these processes lead to an increase in intestinal permeability.
Finally, these processes lead to an increase in intestinal permeability. Thus, leaky gut syndrome develops as a response to pathogens, feed deprivation, and stress.
BIOMARKERS RELATED TO INTESTINAL HEALTH OF ANIMALS
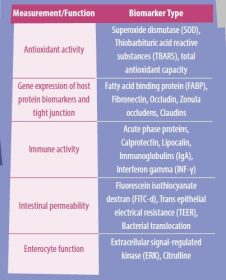
The interactions between the epithelial barrier function, intestinal inflammation, and the microbial environment influence gut health. Therefore, the discovery of reliable, widespread biomarkers to measure intestinal inflammation and barrier function is an important ongoing area of research. A summary of some of the known biomarkers related to intestinal health is presented in Table 1.
- To study intestinal health, it is also important to develop inflammatory gut models with different challenge conditions (anti-nutritional factors, pathogens, toxins, and environmental triggers).
Inflammation can also be associated with oxidative stress and changes in the expression of genes related to oxidative stress, indicating that oxidative stress may have a critical role in the physiological intestinal function.
One quantitative technique that is used to evaluate the integrity of tight junction proteins in epithelial cell monolayers is the measurement of transepithelial electrical resistance (TEER). Mitochondrial respiration is required to maintain TEER, implying that oxidation plays a critical role in Caco-2 cell tight junction stability.
According to Janssen-Duijghuijsen et al., reduced mitochondrial ATP production resulted in a decrease of intestinal permeability and an increase in occludin and claudin-1 gene expression, but a decrease in claudin-2 and claudin-7 gene expression.
- Consequently, a direct connection between mitochondrial function, cellular energy status, and intestinal integrity was established.
Biomarkers of oxidative stress
Often, oxidative stress is quantified by examining metabolites formed during or after an oxidative process.
- An antioxidant enzyme that detoxifies harmful metabolic byproducts and that is usually measured as a biomarker is superoxide dismutase (SOD).
- Other biomarkers that could be used to measure antioxidant activity include thiobarbituric acid reactive substances (TBARS), which are metabolites formed during peroxidation; total antioxidant capacity; and the Griess assay, which utilizes nitrite and nitrate breakdown to determine the concentration of nitric oxide within the cell.
Biomarkers of intestinal health
Biomarkers for the evaluation of intestinal health can also be related to monitoring intestinal function.
- Citrulline is a nitrogen-containing byproduct of glutamine metabolism that can be converted to arginine and is produced mainly by enterocytes of the small intestine.
- Plasma citrulline levels have been associated with intestinal absorption of markers such as mannitol in pre-weaned piglets, indicating that citrulline may be utilized to monitor intestinal function.
- The extracellular signal-regulated kinase (ERK) is another biomarker that can be considered to be an option because it serves as a critical signaling pathway for intestinal epithelial proliferation and tissue healing.
- Thus, serum ERK activity can reflect intestinal disruption caused by a stressor.
Biomarkers related to the immune activity
In the case of biomarkers related to immune activity that can influence intestinal health.
- Secretory IgA (SIgA) is a critical component of the humoral immune system and the leading immunoglobulin that interacts with pathogens on the mucosa surface. Consequently, it has a close relationship with the homeostasis of the intestinal environment.
- A proinflammatory cytokine with immunostimulatory and immunomodulatory properties is interferon-gamma (INF-γ).
- This cytokine has been related to the endocytosis of tight junction proteins. Hence, it has a feasible impact on intestinal permeability.
Ultimately, both innate and adaptive immune responses are likely to provide viable biomarkers for assessing intestinal health.
Histomorphological analysis
The histomorphological analysis is another type of evaluation closely influenced by an adequate balance of the intestinal environment.
- Villus height, crypt depth, and the villus height to crypt depth ratio are parameters that can be used to calculate the area of absorption in the different sections of the intestine, and at the same time be indicative of the epithelial cell turnover in the intestinal barrier.
Biomarkers of intestinal permeability
Bacterial translocation and gene expression of TJ such as claudins, occludins, and zonula occludens (ZO-1) are intestinal permeability biomarkers used to evaluate gut health.
Bacterial translocation has been related to diseases such as chondronecrosis with osteomyelitis in broiler and broiler breeders, suggesting the migration of enteric pathogens to the thoracic vertebrae.
TJs such as occludin have shown to be downregulated in human patients with inflammatory bowel diseases (Crohn’s disease), and in chickens under nutritional gut health challenge condition models, therefore revealing the fundamental role of TJs such as occludin in maintaining intestinal barrier integrity.
A different set of biomarkers candidates include the fatty acid binding proteins (FABP), which are intracellular lipid chaperones in charge of orchestrating lipid metabolism and critical lipid-sensitive pathways in macrophages and adipocytes.
- FABP2 has been studied in humans and in chickens, showing a downregulation response when there is intestinal barrier injury.
Another well-known biomarker that has been utilized in poultry to evaluate intestinal permeability is the measurement of fluorescein isothiocyanate dextran (FITC-d) in the serum.
- During intestinal inflammation, the disruption of the TJ proteins allows the FITC-d molecule to diffuse into systemic circulation, allowing measurement of this biomarker under different challenging conditions, including 24 h of fasting in broiler chickens.

Some non-invasive biomarkers that are currently being studied in fecal samples by different research groups are fibronectin, calprotectin, and lipocalin. These biomarker candidates have shown promising results in chickens; nevertheless, there have been also inconsistencies between studies.
Ultimately, the objective is to continue searching for intestinal health biomarkers that can be easily measured from samples that do not require an extensive preparation time or cost.
Source: avinews.com






