by Daniela Vega Sampedro, Product Manager Organic Acids, EW Nutrition
Feed spoilage is a significant issue for the feed industry, leading to loss of nutrients, feed waste, and substantial economic issues for feed and animal producers worldwide (Leyva Salas et al., 2017). Fungal growth is one of the main causes of feed spoilage; it can occur at any stage of the feed production chain, including grain pre- and post-harvest processes, during feed production or storage. Organic acids and their salts are globally used in animal nutrition for microbial preservation and supporting animal health.
 Organic acids help preserve animal feed and prevent spoilage through molds, yeasts, and mycotoxins
Organic acids help preserve animal feed and prevent spoilage through molds, yeasts, and mycotoxins
The threat of molds and yeasts in animal feed
Yeasts and molds can have both positive and negative effects on products consumed by animals and humans. On the one hand, yeasts are used to produce fermented products, such as bread, wine, and beer. On the other hand, yeasts and molds promote the spoilage of raw materials, food, and feeds (Lowes et al., 2000). Molds are among the most potent food and feed spoilers. They can be very resilient to environmental stress, which is a concern in climate change scenarios (Perrone et al., 2020) and enables them to withstand feed preservation measures (Punt et al., 2020).
Several hundred species of molds and yeasts can invade a large variety of raw materials and feeds. They show an easy adaptation to different environments; for instance, they can grow and reproduce in media with pH levels ranging from 2 to above 9 (Tournas et al., 2001). However, the majority of yeasts and molds require free oxygen to grow and thrive.
Excess moisture, high water activity, and high temperatures in feedstuffs are the main mold growth factors that concern the feed industry (Mohapatra et al., 2017). At storage, grains’ moisture content should not exceed 13%, and the water activity of raw materials, feedstuffs, and finished feed should be maintained below 0.8 (Dijksterhuis et al., 2019). Controlling these points contributes to preventing the growth of most pathogens and undesirable microorganisms.
 Mold growth reduces the nutritional value of feed, which affects animal health and performance
Mold growth reduces the nutritional value of feed, which affects animal health and performance
The microbiology of molds and how they affect the feed
The microbial growth dynamic of grain storage depends on several factors, including the harvest season, grain temperature and moisture content, as well as the type of facility and its environment. For instance, in some areas, grains are harvested at the beginning of the cold season and stored through the following warm season. Storage molds constitute a significant threat to the quality of these raw materials, especially during the warm months, when the stored grains may become hotter than the surrounding environment. This leads to condensation, which increases moisture and water activity. Molds easily thrive in these conditions.
Storage molds reduce the nutritional and commercial value of grains and feeds. For grains, their commercial value decreases when the appearance of kernels changes in a manner recognized by the grain industry as kernel damage. The chemical composition of feeds may deteriorate due to enzymatic actions, resulting in a loss of nutrients (energy, vitamins) and the production of free fatty acids and other unwanted by-products (Reed et al., 2007).
Extensive research has established the factors that influence mold-induced deterioration during grain storage and which management strategies are required:
- Moisture content and water activity (a function of the temperature, moisture content, and substrate) – Microorganisms have a limiting water activity below which they cannot grow; therefore, drying the grains below that critical level is part of an effective mold control strategy (Mannaa & Kim, 2017).
- Temperature – Grain-contaminating molds thrive in tropical regions, where high temperature and humidity conditions predominate. In general, molds are inactive if the grains are stored below 20 °C (Mousa et al., 2013). However, the temperature of stored grains increases as molds begin to grow in the warmer and/or wetter parts of the grain/feed mass and feed, and heat is generated due to respiration, accelerating the deterioration rate. Moreover, the presence of a temperature gradient in the feedstuffs causes air to move, accelerating the transfer of moisture to cooler grain (Mannaa & Kim, 2017).
- Grain quality, including previous storage conditions, insect infestation, presence of broken kernels, and impurities – When grain is too warm, the rate of insects’ breeding is higher (they respond to higher temperatures), the grain contains more humidity and may carry fungal spores. Broken kernels are an easier target for mold and insect infestations than whole ones, increasing the possibility of spoilage (Marcos Valle et al., 2021).
- Duration of storage, management, and aeration influence the oxygen and carbon dioxide concentration in the grain mass, which plays a role in mold growth (Marcos Valle et al., 2021).
The consequences of storage deterioration include:
- worse organoleptic properties (aspect, texture, taste, and aroma) of grains and feeds
- more kernel damage,
- higher fat acidity,
- slight increase in protein content as non-protein constituents are consumed by mold respiration, causing
- lower energy value of the grain/feed (Reed et al., 2007), and
- lower content of vitamins A, B1, D3, E, and K.
Molds and mycotoxins: a toxic relationship for animal health
Beyond their negative impact on feed quality, some fungal genera such as Aspergillus, Penicillium, Alternaria, and Fusarium can produce mycotoxins, secondary metabolites that have toxic effects on humans and animals (Greco et al., 2015). Roughly 60% of raw materials produced for agriculture purposes worldwide are estimated to be contaminated by fungi and mycotoxins (Eskola et al., 2020). Mycotoxins can induce toxic, carcinogenic, and mutagenic reactions even at low concentrations. Their presence in the final feed is a sign of alert as, usually, these metabolites are resistant to technological treatments. Thus, it is important to stop them from entering the feed production chain (Leyva Salas et al., 2017).
 Feed-contaminating Fusarium species produce mycotoxins such as trichothecenes, zearalenone, and Fumonisin.
Feed-contaminating Fusarium species produce mycotoxins such as trichothecenes, zearalenone, and Fumonisin.
Organic acids: Unrivaled in preventing feed spoilage
It is crucial to reduce the feed losses and improve animal health by controlling fungal contamination at all stages of the feed production chain: from pre-harvest strategies on the field to post-harvest management during storage and even at feed processing. Throughout these processes, producers can apply different management practices. For instance, in field crops, fungal growth can be prevented through crop rotation and tillage; the use of fungicides is a later measure when mold presence exceeds critical levels.
Post-harvest management of grains and their by-products includes drying and storage management through moisture and temperature monitoring and aeration programs. Other spoilage-prevention measures include good hygiene practices and thermal treatments in feed production. However, feed producers and farmers face limitations in applying and linking such measures to tackle the occurrence of these undesirable pathogens (Dijksterhuis et al., 2019).
Certain organic acids, such as propionic, sorbic, benzoic, and acetic acids, have proven effective in preventing mold growth and feed spoilage. These organic acids are used globally now, not only for improving animal nutrition but also for supporting animal health (Dijksterhuis et al., 2019).
Pro-Stabil BSL is a product that harnesses the feed preservation effects of organic acids and combines them with surfactants. This means that it can offer a strong yeast and mold inhibition while maintaining the moisture in feed, thus reducing the risk of microbial challenges while prolonging the shelf life of feedstuffs and compound feeds.
Trial results: Pro-Stabil BSL is a great tool to reduce mold growth and manage moisture
Pro-Stabil BSL contains a synergistic blend of organic acids and a surfactant that leads to
» Improved moisture dispersion in the feed
» Increased water retention (reduced water activity)
» Improved anti-mold agent dispersion in the feed and grain
Trial results show a significant decrease in mold growth when Prostabil BSL was added to compound feed. In addition, when moisture was added at 2%, moisture from the environment was also observed, but the mold counts still decreased (Figure 1).
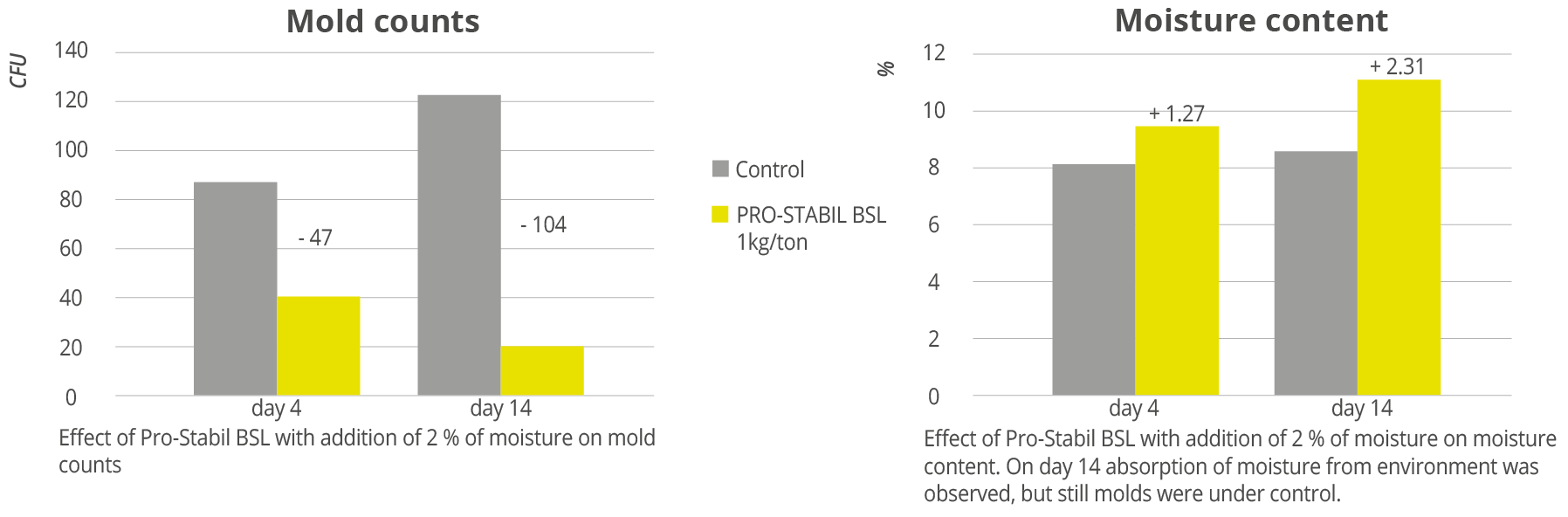 Figure 1: Effects of Pro-Stabil BSL with addition of 2 % moisture on feed quality indicators
Figure 1: Effects of Pro-Stabil BSL with addition of 2 % moisture on feed quality indicators
When adding Pro-Stabil BSL to animal feed, the following benefits can be expected:
- Reduction and prevention of mold growth and recontamination
- Improved moisture management
- Improved feed mill efficiency production
- Improved microbiological quality of grains and feed
- Shrinkage management by increasing moisture in feed with no risk of mold development
- Reduced water dissipation
Mold growth can lead to sensory defects in feed and reduce its nutritional value. It can also harm animals through the production of mycotoxins. Pro-Stabil BSL offers a safe solution that is also easy to handle. Using the preservative properties of organic acids, Pro-Stabil BSL helps to reduce feed spoilage and its associated effects on animal health and performance.
References
Dijksterhuis, Jan, Martin Meijer, Tineke van Doorn, Jos Houbraken, and Paul Bruinenberg. “The Preservative Propionic Acid Differentially Affects Survival of Conidia and Germ Tubes of Feed Spoilage Fungi.” International Journal of Food Microbiology 306 (2019): 108258. https://doi.org/10.1016/j.ijfoodmicro.2019.108258.
Eskola, Mari, Gregor Kos, Christopher T. Elliott, Jana Hajšlová, Sultan Mayar, and Rudolf Krska. “Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%.” Critical Reviews in Food Science and Nutrition 60, no. 16 (2020): 2773-2789. https://doi.org/10.1080/10408398.2019.1658570
Greco, Mariana, Minna Kemppainen, Graciela Pose, and Alejandro Pardo. “Taxonomic Characterization and Secondary Metabolite Profiling Of Aspergillus Section Aspergillus Contaminating Feeds And Feedstauffs.” Toxins 7, no. 9 (2015): 3512–37. https://doi.org/10.3390/toxins7093512.
Harein, P., & Meronuck, R. (1995). Stored grain losses due to insects and molds and the importance of proper grain management. In V. Krischik, G. W. Cuperus, & D. Galliart (Eds.), Stored product management (pp. 29e31). Oklahoma Cooperative Extension Service Publication. E-912.
Leyva Salas, Marcia, Jérôme Mounier, Florence Valence, Monika Coton, Anne Thierry, and Emmanuel Coton. “Antifungal Microbial Agents for Food Biopreservation—a Review.” Microorganisms 5, no. 3 (2017): 37. https://doi.org/10.3390/microorganisms5030037.
Lowes, K. F., C. A. Shearman, J. Payne, D. MacKenzie, D. B. Archer, R. J. Merry, and M. J. Gasson. “Prevention of Yeast Spoilage in Feed and Food by the Yeast Mycocin Hmk.” Applied and Environmental Microbiology 66, no. 3 (2000): 1066–76. https://doi.org/10.1128/aem.66.3.1066-1076.2000.
Mannaa, Mohammed, and Ki Deok Kim. “Influence of temperature and Water activity on Deleterious fungi AND Mycotoxin production during grain storage.” Mycobiology 45, no. 4 (2017): 240–254. https://doi.org/10.5941/myco.2017.45.4.240.
Marcos Valle, F. J., Castellari, C., Yommi, A., Pereyra, M. A., & R. Bartosik. “Evolution of grain microbiota during hermetic storage of corn (zea mays l.).” Journal of Stored Products Research 92 (2021): 101788. https://doi.org/10.1016/j.jspr.2021.101788.
Mohapatra, D., Kumar, S., Kotwaliwale, N., and K. K. Singh. “Critical factors responsible for fungi growth in stored food grains and non-Chemical approaches for their control.” Industrial Crops and Products 108 (2017): 162–182. https://doi.org/10.1016/j.indcrop.2017.06.039.
Mousa, W., Ghazali, F. M., Jinap, S., Ghazali, H. M., and S. Radu. “Modeling growth rate and assessing AFLATOXINS production by Aspergillus flavusas a function of Water activity and temperature on polished and brown rice.” Journal of Food Science 78, no. 1 (2013). https://doi.org/10.1111/j.1750-3841.2012.02986.x.
Perrone G, Ferrara M, Medina A, Pascale M, and N. Magan. “Toxigenic Fungi and Mycotoxins in a Climate Change Scenario: Ecology, Genomics, Distribution, Prediction and Prevention of the Risk.” Microorganisms 8, no. 10 (2020): 1496. https://doi.org/10.3390/microorganisms8101496.
Punt, Maarten, Tom van den Brule, Wieke R. Teertstra, Jan Dijksterhuis, Heidy M.W. den Besten, Robin A. Ohm, and Han A.B. Wösten. “Impact of Maturation and Growth Temperature on Cell-size Distribution, Heat-Resistance, Compatible Solute Composition and Transcription Profiles of Penicillium Roqueforti Conidia.” Food Research International 136 (2020): 109287. https://doi.org/10.1016/j.foodres.2020.109287.
Reed, Carl, Stella Doyungan, Brian Ioerger, and Anna Getchell. “Response of Storage Molds to Different Initial Moisture Contents of Maize (Corn) Stored AT 25°C, and Effect on Respiration Rate and Nutrient Composition.” Journal of Stored Products Research 43, no. 4 (2007): 443–58. https://doi.org/10.1016/j.jspr.2006.12.006.
Tournas, Valerie, Michael E. Stack, Phillip B. Mislivec, Herbert A. Koch, and Ruth Bandler. “Bacteriological Analytical Manual Chapter 18: Yeasts, Molds and Mycotoxins.” U.S. Food and Drug Administration. April 2001. https://www.fda.gov/food/laboratory-methods-food/bam-chapter-18-yeasts-molds-and-mycotoxins.







