Source: PoultryHealthToday.com
Extensive screening of more than 40 vaccine prototypes and a unique construction are responsible for the strong, early protection Poulvac® Procerta® HVT-IBD-ND provides against three important diseases of poultry.
“Poulvac Procerta HVT-IBD-ND is the culmination of meticulous research conducted by a team of our top molecular scientists using the most advanced technology available,” said Sing Rong, PhD, research director at Zoetis.
The backbone of the vaccine is herpesvirus of turkey (HVT), an avirulent virus that replicates in chickens and stimulates immunity against Marek’s disease (MD). Well known as a useful vector for delivering major avian pathogens, the HVT backbone includes genetic insertions that initiate immunity against infectious bursal disease (IBD) and Newcastle disease (ND) viruses.
Boosts immunogenicity
“Our novel vector insertion site on the HVT genome and a strong promoter that boosts immunogenicity are two important factors that set the vaccine apart from other vaccines in the same category,” Rong said, noting that the insertion site is proprietary and patented.
In addition, the technique used to produce the vaccine requires fewer passages during recombinant construction than was possible in the past. This is important, she explained, because it’s well known that MD vaccines can lose immunogenicity the more times they are passaged.
When developing the vaccine, Zoetis scientists started by constructing 43 recombinant prototypes. They then subjected the vaccine prototypes to rigorous testing to find the one that provided the best, early immunity — a requirement of utmost importance based on field feedback from Zoetis veterinarians, she said. The prototype finally selected is the vaccine that is now Poulvac Procerta HVT-IBD-ND.
IBDV immunity at 14 days of age
To determine the new vaccine’s onset of immunity against IBDV, investigators at Zoetis challenged birds that had been vaccinated in ovo with a virulent, classic strain of the virus via eye drop at 14 and 21 days of age. Four days after each challenge, they evaluated efficacy by examining the birds for bursal lesions.
Results indicated that 90% of the birds that received Poulvac Procerta HVT-IBD-ND were protected after the 14-day-old and 21-day-old challenge. Among unvaccinated, challenged controls, 0% and 5% were protected after the 14-day and 21-day challenge, respectively, Rong said.1
Efficacy at 34 days was 100%, whether the vaccine was delivered in ovo or subcutaneously at hatch,2,3 she said.“
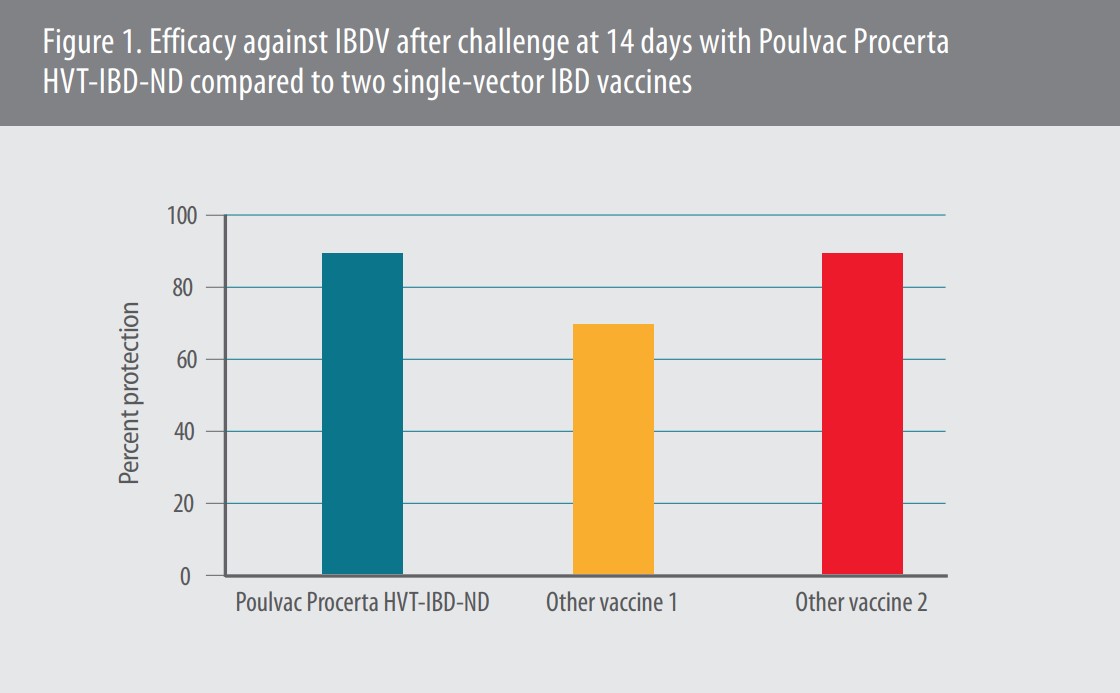
Our team wanted to make sure each component of the new, trivalent vaccine was as effective against IBDV as single-vector vaccines,” she continued. Compared to two other commercial HVT-IBD vaccines, Poulvac Procerta HVT-IBD-ND was just as effective as one — providing 90% protection — and better than the other, which provided only 70% protection after the 14-day-old challenge (Figure 1).4
Virulent ND, MD protection
The efficacy of the new vaccine against virulent (velogenic) ND was determined by clinical signs and mortality, Rong said.
Administered in ovo, the vaccine demonstrated 75% protection after a challenge at 14 days of age and 95% protection after challenge at 21 days of age.5 None of the unvaccinated, control birds were protected, confirming the validity of the challenge.
When birds that received the vaccine subcutaneously at hatch or in ovo were challenged at 28 days of age, 95% to 100% were protected.6,7
Zoetis scientists also tested the new vaccine for protection against virulent MDV. Poulvac Procerta HVT-IBD-ND provided 80% and 83% protection, respectively, as early as 5 days of age after receiving the vaccine in ovo8 or subcutaneously at hatch.9
Duration of immunity at least 63 days
The long duration of immunity with HVT vaccines is well established, and Poulvac Procerta HVT-IBD-ND is no exception, Rong said. When birds that received the new vaccine were challenged at 63 days of age with classic IBDV, very virulent IBDV or with virulent NDV, 100% were protected.10,11,12,13
HVT vaccines are also known to be safe and do not cause reactions like those that can occur with conventional live vaccines.
Early protection essential
Kalen Cookson, DVM, director, clinical research, Zoetis, said early protection provided by the vaccine is essential to avoiding costly losses associated with all three diseases.
IBDV, which is highly contagious, tends to strike young birds and can lead to severe, permanent immunosuppression and vulnerability to costly secondary bacterial infections. Maternal antibody levels against the virus generally start to wane around 14 days of age, which is why a vaccine that initiates protection at about the same time is especially valuable, he said.
Meanwhile, a long duration of immunity against IBDV is also important, he added, since the virus can affect older birds, leading to temporary immunosuppression.
MD virus is another pathogen that causes immunosuppression and predisposes birds to secondary infections. In older birds, it can cause tumors that lead to condemnations, Cookson continued.
Velogenic ND can be highly fatal. Although velogenic ND is currently rare in the US,14 this vaccine can be very useful in high-challenge areas or where even less-pathogenic strains can cause production losses and complicated respiratory disease. Even when ND challenge is not consistently high, Poulvac Procerta HVT-IBD-ND may help producers more effectively control infectious bronchitis (IB) by allowing them to avoid live ND vaccine competition with their live IB vaccine program, he said.
“The ability to effectively provide robust, early protection against three important diseases with one vaccine is an exciting development for the poultry industry,” Cookson said.
“When used with the proven technology of the Embrex® Inovoject® system and the technical support Zoetis is known for, Poulvac Procerta HVT-IBD-ND brings tremendous safety, efficacy and convenience to producers all in one vaccine.”
1 Data on file, Study Report No. B812W-US-18-A07, Zoetis LLC.
2 Data on file, Study Report No. B812R-US-19-A80, Zoetis LLC.
3 Data on file, Study Report No. B812R-US-19-A81, Zoetis LLC.
4 Data on file, Study Report No. B812W-US-18-A07, Zoetis LLC.
5 Data on file, Study Report No. B812W-US-18-A06, Zoetis LLC.
6 Data on file, Study Report No. B812R-US-19-A85, Zoetis LLC.
7 Data on file, Study Report No. B812R-US-19-A84, Zoetis LLC.
8 Data on file, Study Report No. B812R-US-20-D96, Zoetis LLC.
9 Data on file, Study Report No. B812R-US-18-A64, Zoetis LLC.
10 Data on file, Study Report No. B814R-US-19-C18, Zoetis LLC.
11 Data on file, Study Report No. B814R-US-20-D45, Zoetis LLC.
12 Data on file, Study Report No. B814R-US-20-D10, Zoetis LLC.
13 Data on file, Study Report No. B814R-US-20-C74, Zoetis LLC.
14 USDA virulent Newcastle Disease. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease[1]information/avian/virulent-newcastle/vnd Accessed October 29, 2021
All trademarks are the property of Zoetis Services LLC or a related company or a licensor unless otherwise noted.
DISCOVERIES, Issue 15
Discoveries is a series of research news reports written by the editors of Poultry Health Today on behalf of the US Poultry Business of Zoetis.
DLV-00021
July2022